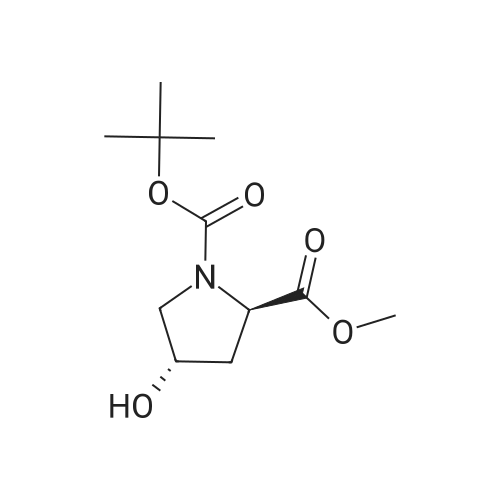
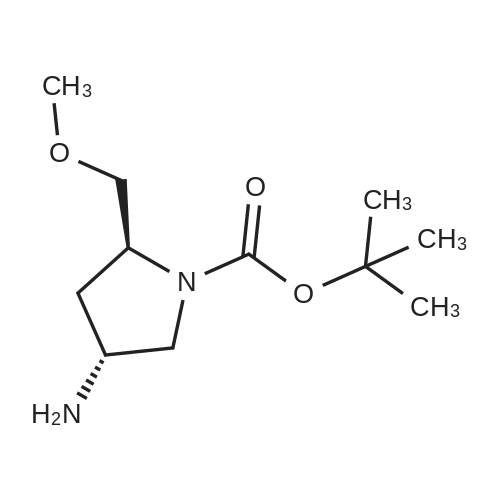
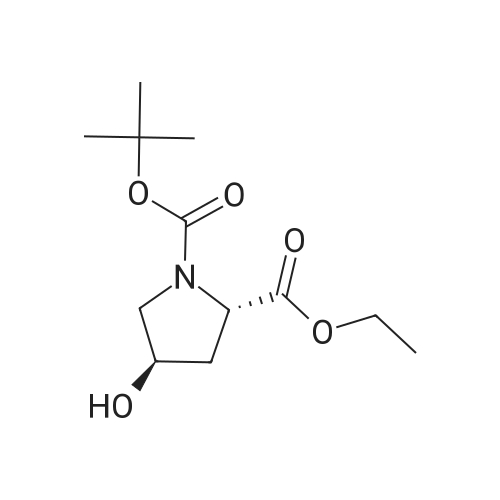
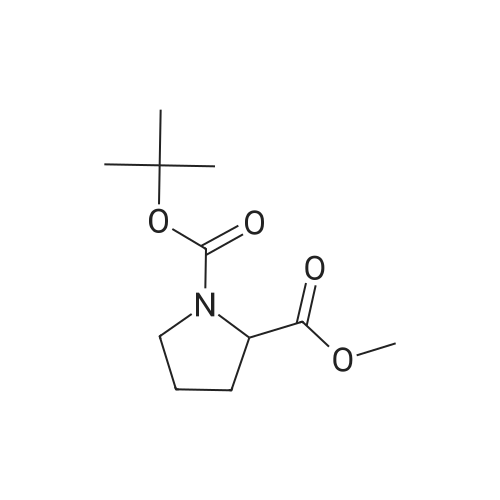
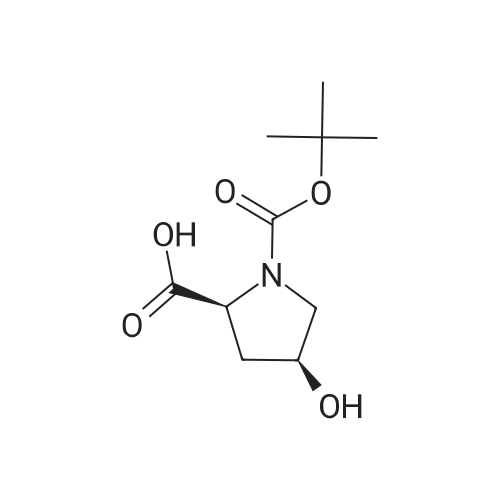
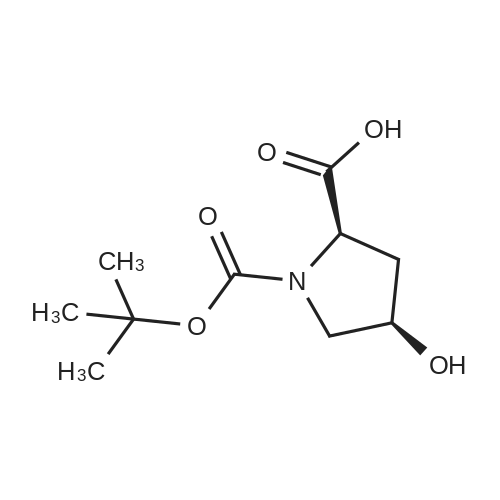
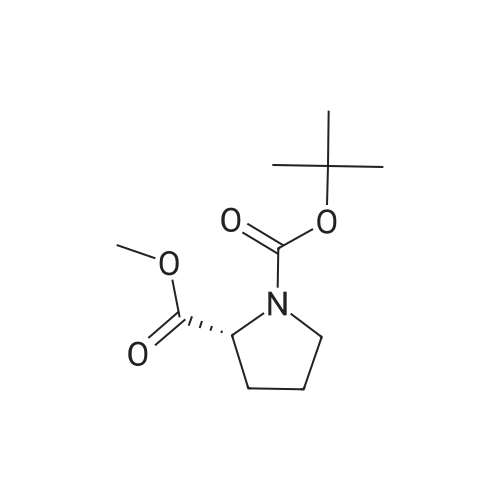
|
Multi-step reaction with 2 steps
1: 94 percent / PPh3, DEAD
2: 95 percent / NaOMe / methanol |
|
|
Multi-step reaction with 2 steps
1: 66 percent / triphenylphosphine, diethyl azodicarboxylate / tetrahydrofuran / 0.67 h / 0 °C
2: 89 percent / aq. NaOH / methanol / 0.33 h |
|
|
With triphenylphosphine; diethylazodicarboxylate; 4-nitro-benzoic acid In tetrahydrofuran at 0 - 30℃; |
2.B
Step. The compound of Step A (144. 5g, 0. 59 mol), triphenyl phosphine (200g, 0. 76 mol, 1. 29 eq), p- nitrobenzoic acid (150 g, 0. 9 mol, 1. 52 eq) and THF (2. 5 L) were mixed and cooled to 0°C. Diethyl diazodicarboxylate (120 mL, 0. 76 mol, 1. 29 eq) was added over 10 min. The reaction exotherme to 30°C and was allowed to cool to room temperature and stir overnight. The reaction was concentrated, the residue diluted with ether (1L) and the resulting suspension filtered. The filtrate was again concentrated, re-dissolved in CH2C12 and chromatographed (silica, linear gradient CH2C12 to 5% EtOAc/CH2Cl). The product cuts were combined and concentrated. The concentrate was flushed with ether then hexanes to afford a white slurry which was filtered and dried giving 218. 7g of the title compound as a white solid. |
|
Multi-step reaction with 2 steps
1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran
2: lithium hydroxide monohydrate; lithium hydroxide monohydrate / tetrahydrofuran; methanol |
|
|
Multi-step reaction with 2 steps
1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran / 0 - 20 °C / Inert atmosphere
2: methanol; potassium carbonate / 1 h / 20 °C / Inert atmosphere |
|
|
Multi-step reaction with 3 steps
1: triethylamine / dichloromethane / 2 h / 0 °C
2: (methylsulfinyl)methane / 6 h / 20 - 90 °C
3: potassium carbonate; methanol / 0.5 h / 0 - 20 °C |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 6.5 h / 0 - 20 °C / Inert atmosphere
2: Caswell No. 744A / propan-2-one / 14 h / Reflux |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; triethylamine; di-isopropyl azodicarboxylate / tetrahydrofuran / 6 h / 0 - 20 °C
2: Caswell No. 744A / propan-2-one / 14 h / Reflux |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; diethylazodicarboxylate / toluene; tetrahydrofuran / -4 - 20 °C
2: anhydrous sodium carbonate; methanol / 4 h / 20 °C |
|
|
Stage #1: 1-(tert-butyl) 2-methyl (2S,4R)-4-hydroxypyrrolidine-1,2-dicarboxylate With methanesulfonyl chloride; triethylamine In dichloromethane
Stage #2: With benzoic acid sodium salt In dichloromethane at 90℃;
Stage #3: With potassium carbonate In methanol; dichloromethane |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; diethylazodicarboxylate / toluene; tetrahydrofuran / -4 - 20 °C
2: anhydrous sodium carbonate / methanol / 4 h / 20 °C |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; di-isopropyl azodicarboxylate / dichloromethane / 4 h / 0 - 20 °C / Inert atmosphere
2: methanol; Caswell No. 744A / 4 h / 40 °C |
|
|
Multi-step reaction with 2 steps
1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 48 h / 0 - 50 °C / Inert atmosphere
2: Caswell No. 744A / methanol / 40 h / 40 °C / Inert atmosphere |
|
|
Multi-step reaction with 2 steps
1.1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran / 0 - 20 °C / Inert atmosphere; Cooling with ice bath
2.1: methanol; potassium hydroxide / 0.5 h / 0 °C / Cooling with ice bath; Inert atmosphere
2.2: Inert atmosphere |
|
|
Multi-step reaction with 3 steps
1.1: triethylamine / tetrahydrofuran / 16 h / 0 - 20 °C
2.1: Caswell No. 744A / N,N-dimethyl-formamide / 16 h / 70 °C
3.1: benzoic acid sodium salt / (methylsulfinyl)methane / 17 h / 90 °C
3.2: 1 h / 20 °C |
|
|
Multi-step reaction with 2 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; trichloroisocyanuric acid / dichloromethane / 2 h / 0 - 25 °C / Large scale
2: sodium tetrahydridoborate |
|
|
Multi-step reaction with 2 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; trichloroisocyanuric acid / dichloromethane / 2 h / 0 - 25 °C / Large scale
2: D-glucose; NADP; glucose dehydrogenase; keto reductase KRED PK076 / aq. phosphate buffer / 20 h / 37 °C / Large scale; Enzymatic reaction |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping