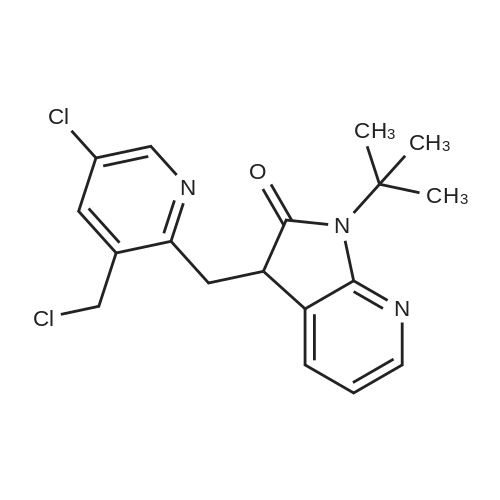
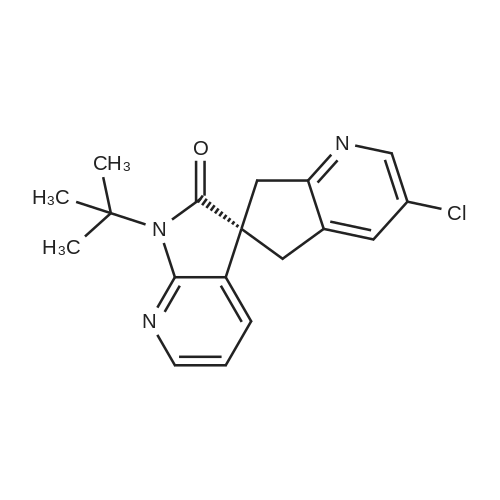
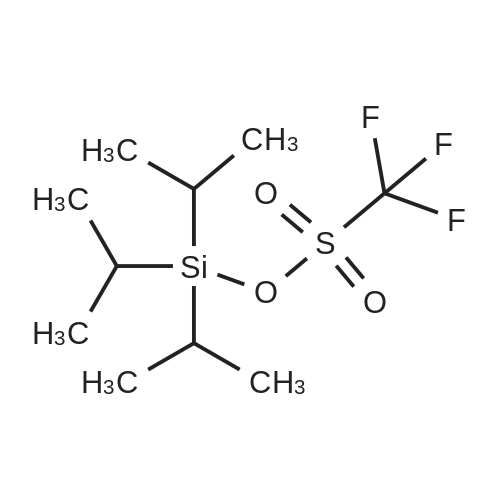
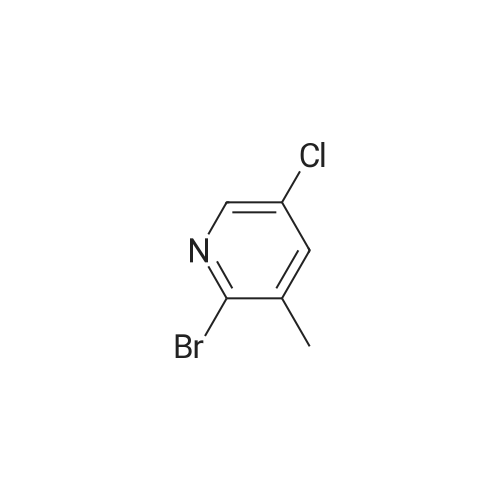
| 90% |
With sulfuric acid; In tetrahydrofuran; at 20℃; for 1h; |
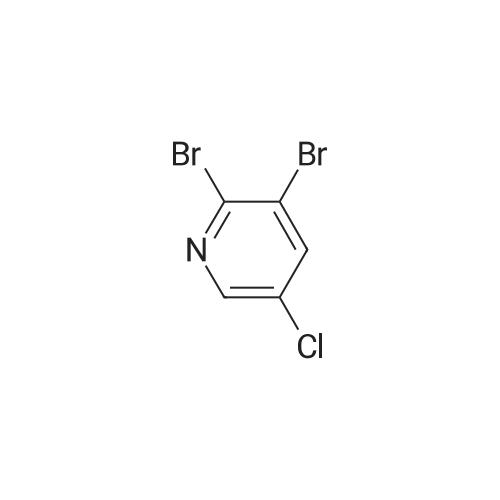
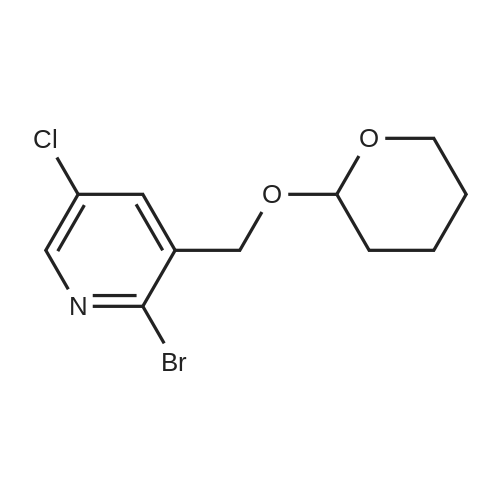
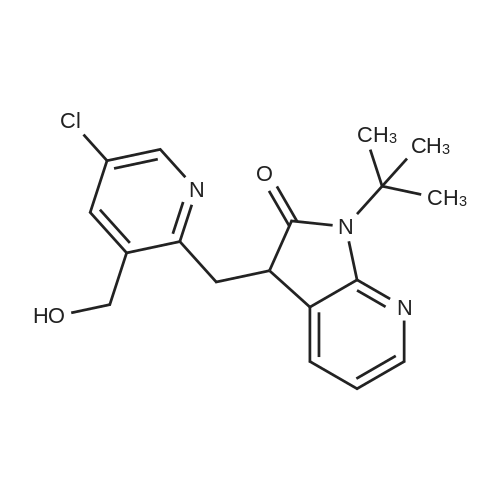
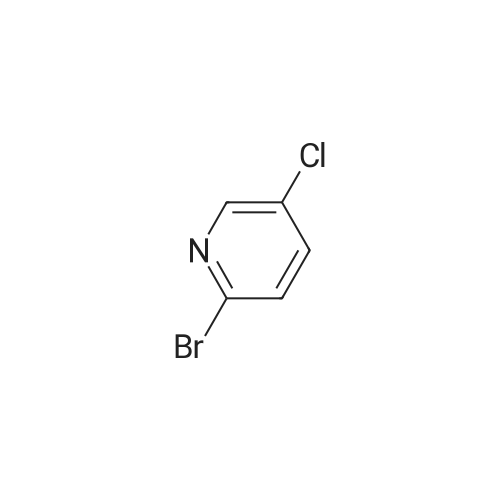
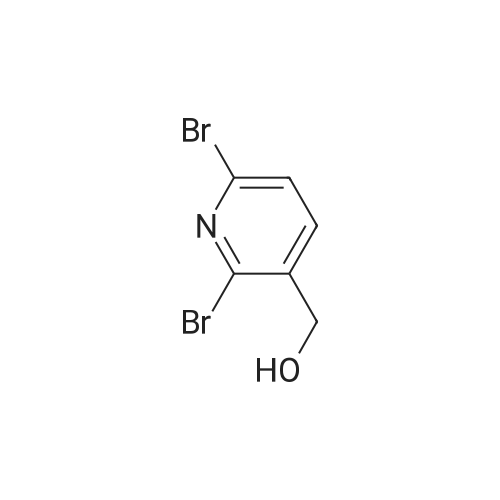
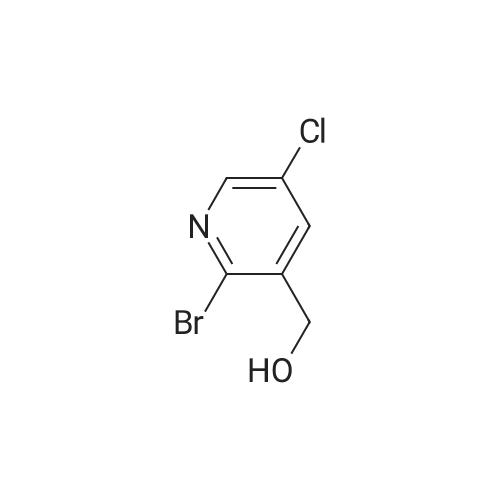
To a solution of compound 47.2 (7.30 g, 33.0 mmol) in tetrahydrofuran (30 mL) was added3,4-dihydro-2H-pyran (3.30 g, 39.6 mmol) and concentrated sulfuric acid (185 mg) at room temperature. The solution mixture was stirred for 1 h, concentrated and purified directly via flash silica chromatography (5-10% ethyl acetate/petrol ether) to provide 2-bromo-5-chloro- 3-(tetrahydropyran-2-yloxymethyl)pyridine (9.10 g, 90%) as a colourless oil. 1H NMR(ODd3, 400 MHz) O 1.76 (m, 6H), 3.63 (m, 1H), 3.89 (m, 1H), 4.51 (m, 1H), 4.81 (m, 2H),7.85 (5, 1H), 8.27 (5, 1H). LCMS (305.9 [M+H]). |
|
With sulfuric acid; In 2-methyltetrahydrofuran; at 20℃; for 0.166667h; |
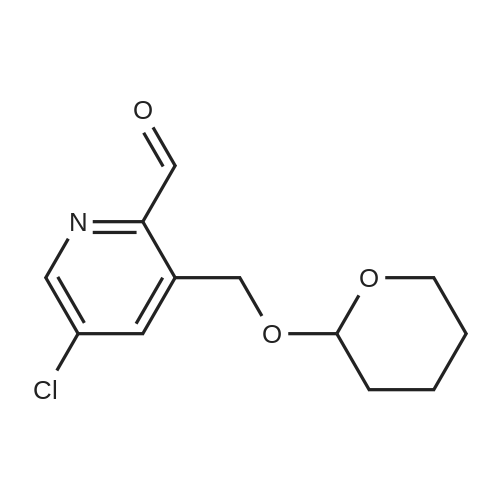
To a solution of 1 (5.0 g, 22.5 mmol) in 2-MeTHF (15 mL) was added 3,4- dihydro-2H-pyran (2.7 mL, 29.6 mmol) and concentrated sulfuric acid (125 mg) at room temperature. The solution was stirred for 10 min and was then cooled to - 3 C. Isopropylmagnesium chloride lithium chloride solution (1.3 M, 30 ml, 39 mmol) was slowly added at -3 to 3 C. The resulting solution was stirred at -3 C for 3 h until a HPLC showed the conversion was greater than 97%. DMF (5 ml) was added over 15 min below 5 C. The resulting solution was stirred for another 1 h at this temperature. The reaction mixture was quenched by addition of MTBE (50 mL), 15% aqueous citric acid (25 mL) and water (15 mL). The organic layer was separated and washed with 5% aqueous NaCl (50 mL) twice. The organic solution was concentrated under vacuum at 50 C to give 2 as an oil (6.2 g, 68 wt%, 16.6 minol, 74% yield). The crude product was used directly for the next step without further purification. The pure sample was isolated by flash chromatography on silica gel with 5% ethyl acetate in hexane as eluants. NMR (CDC13, 400 MHz): 8 10.13 (s, 1H), 8.65 (s, 1H), 8.20 (s, 1H), 5.25 (d, J = 16.6 Hz, 1H), 5.01 (d, J= 16.6 Hz, 1H), 4.80 (m, 1H), 3.88 (m, 1H), 3.58 (m, 1H), 1.7 (m, 6H); 13C NMR (CDC13, 100 MHz): 8 194.20, 147.06, 146.32, 138.98, 136.41, 134.87, 99.04, 64.42, 62.72, 30.53, 25.30, 19.66. |
|
|
To a solution of 52 (5.0 g, 22.5 mmol) in 2-MeTHF (15 mL) was added 3,4-dihydro-2H-pyran (2.7 mL, 29.6 mmol) and concentrated sulfuric acid (125 mg) at room temperature. The solution was stirred for 10 mm and was then cooled to - 3 C. Isopropylmagnesium chloride lithium chloride solution (1.3 M, 30 ml, 39 mmol) was slowly added at -3 to 3 C. The resulting solution was stirred at -3 C for 3 h until a HPLC showed the conversion was greater than 97%. DMF (5 ml) was added over 15 mm below 5 C. The resulting solution was stirred for another 1 h at this temperature. The reaction mixture was quenched by addition of MTBE (50 mL), 15% aqueous citric acid (25 mL) and water (15 mL). The organic layer was separated and washed with 5% aqueous NaCl (50 mL) twice. The organic solution was concentrated under vacuum at 50 C to give 53 as an oil (6.2 g, 68 wt%, 16.6 mmol, 74% yield). The crude product was used directly for the next step without further purification. The pure sample was isolated by flash chromatography on silica gel with 5% ethyl acetate in hexane as eluants. 1H NMR (CDCl3, 400 MHz): oe 10.13 (s, 1H), 8.65 (s, 1H), 8.20 (s, 1H), 5.25 (d, J= 16.6 Hz, 1H), 5.01 (d,J= 16.6 Hz, 1H), 4.80 (m, 1H), 3.88 (m, 1H), 3.58 (m, 1H), 1.7 (m, 6H);13c NMR (CDCl3, 100 MHz): oe 194.20, 147.06, 146.32, 138.98, 136.41, 134.87, 99.04, 64.42, 62.72, 30.53, 25.30, 19.66. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping