|
With triethylamine; In chloroform; at 0℃; for 1.1h; |
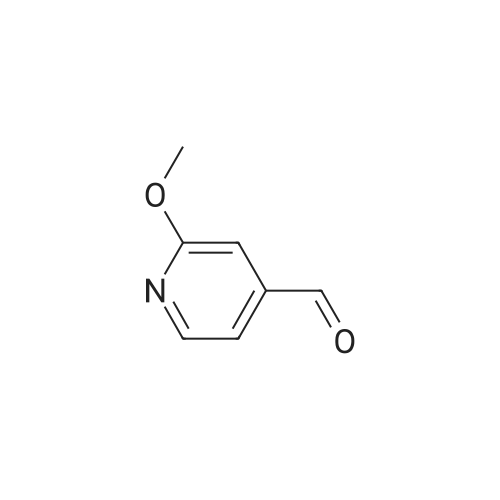
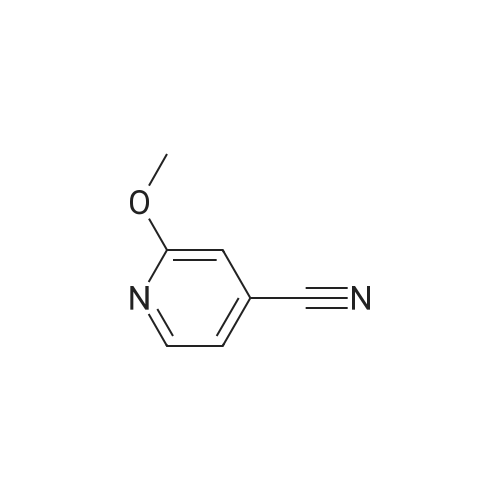
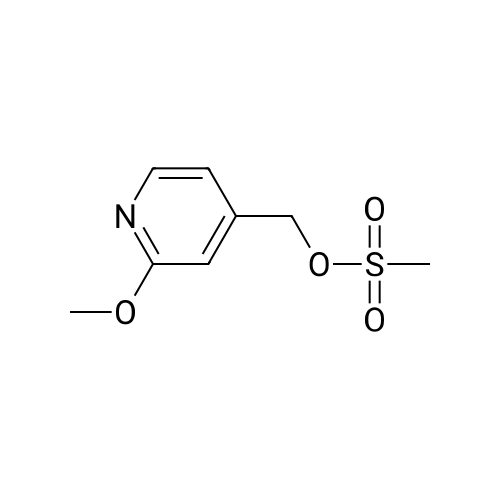
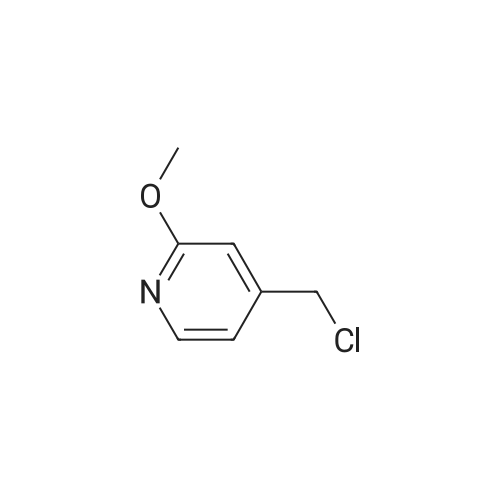
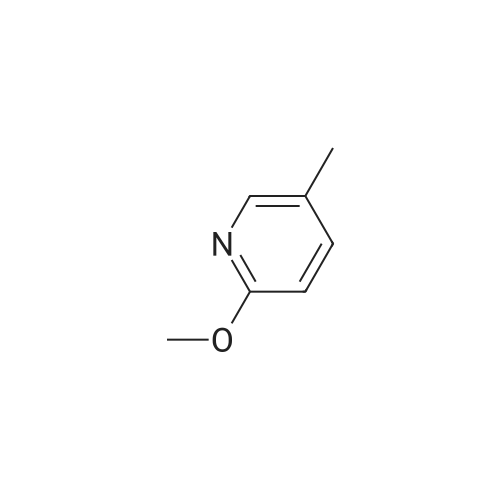
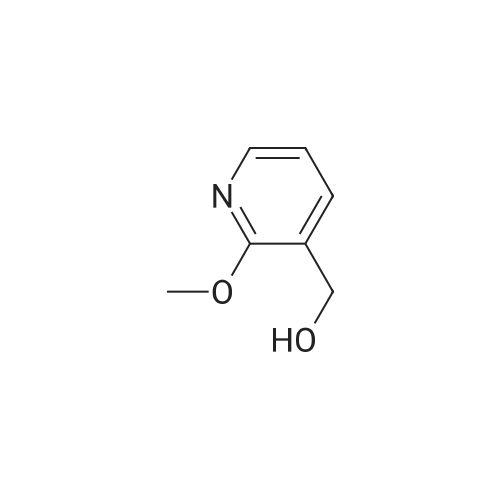
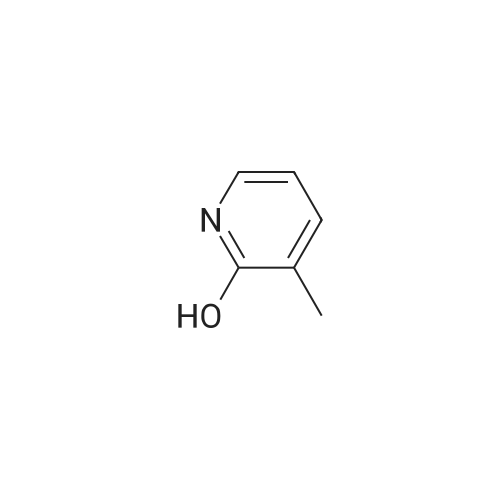
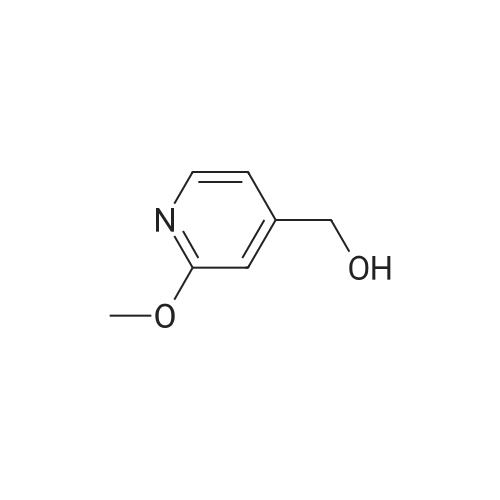
Example X; To a stirred solution of <strong>[123148-66-3](2-Methoxy-pyridin-4-yl)-methanol</strong> (155 mg, 1 mmol) in chloroform (10 ml) cooled in an ice bath under nitrogen atmosphere, was added triethylamine (210 mul, 1.5 mmol) and methanesulfonyl chloride (90 mul, 1.2 mmol) was added dropwise. After stirring for 1.1 hr., the reaction mixture was washed with saturated aqueous sodium bicarbonate, dried with anhydrous magnesium sulfate, filtered, and evaporated in vacuo. The residue was further dried in high vacuo for ca. 20 min. and mixed with 6-(3,5-Dimethyl-benzoyl)-5-isopropyl-1H-pyrimidine-2,4-dione (20) (286 mg, 1 mmol), powdered anhydrous potassium carbonate (138 mg, 1 mmol), and lithium iodide (134 mg, 1 mmol). DMF (5 ml) was then added to the mixture at room temperature and stirred for overnight. After evaporation of DMF, the residue was dissolved in methanol-chloroform (1:9) and filtered through celite pad. The filtrate was then evaporated in vacuo and the residue was purified by silica gel column chromatography (eluent, EA:hexanes (1:2)) to afford 160 mg (38%) of a white solid. m.p. 166-167 C.; 1H-NMR (200 MHz, CDCl3) delta 1.13(3H, d, J=6.7 Hz), 1.22 (3H, d, J=6.7 Hz), 2.26 (1H, m), 3.82 (3H, s), 4.54 (1H, d, J=16.0 Hz), 4.94 (1H, d, J=16.0 Hz), 6.36 (1H, s), 6.56 (1H, d, J=5.3 Hz), 7.23 (1H, s), 7.34 (2H, s), 7.93 (1H, d, J=5.3 Hz), 9.30 (1H, s); m/z (EI) 407(M+); HRMS (EI) Calcd, 407.184662, Found 407.184507.; Example AE; To a stirred solution of <strong>[123148-66-3](2-Methoxy-pyridin-4-yl)-methanol</strong> (155 mg, 1 mmol) in chloroform (10 ml) cooled in an ice bath under nitrogen atmosphere, was added triethylamine (210 mul, 1.5 mmol) and methanesulfonyl chloride (90 mul, 1.2 mmol) was added dropwise. After stirring for 1.1 hr., the reaction mixture was washed with saturated aqueous sodium bicarbonate, dried with anhydrous magnesium sulfate, filtered, and evaporated in vacuo. The residue was further dried in high vacuo for ca. 20 min. and mixed with 6-(3-Chloro-5-methyl-benzoyl)-5-isopropyl-1H-pyrimidine-2,4-dione (50) (306 mg, 1 mmol), powdered anhydrous potassium carbonate (138 mg, 1 mmol), and lithium iodide (134 mg, 1 mmol). DMF (5 ml) was then added to the mixture at room temperature and stirred for overnight. After evaporation of DMF, the residue was dissolved in methanol-chloroform (1:9) and filtered through celite pad. The filtrate was then evaporated in vacuo and the residue was purified by silica gel column chromatography (eluent, EA:hexanes (1:2)) to afford 160 mg (37%) of a white solid. m.p. 98-101 C.; 1H-NMR (200 MHz, CDCl3) delta 1.12(3H, d, J=7.0 Hz), 1.21 (3H, d, J=7.0 Hz), 2.21-2.38 (4H, m), 3.82 (3H, s), 4.47 (1H, d, J=16.0 Hz), 5.05 (1H, d, J=16.0 Hz), 6.34 (1H, s), 6.54 (1H, d, J=5.3 Hz), 7.33 (1H, s), 7.37 (1H, s), 7.52 (1H, s), 7.92 (1H, d, J=5.3 Hz), 8.90 (1H, s); m/z (EI) 427(M+); HRMS (EI) Calcd, 427.129553, Found 427.129884. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping