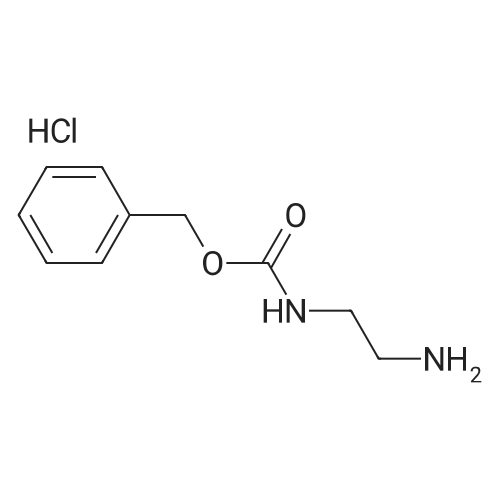
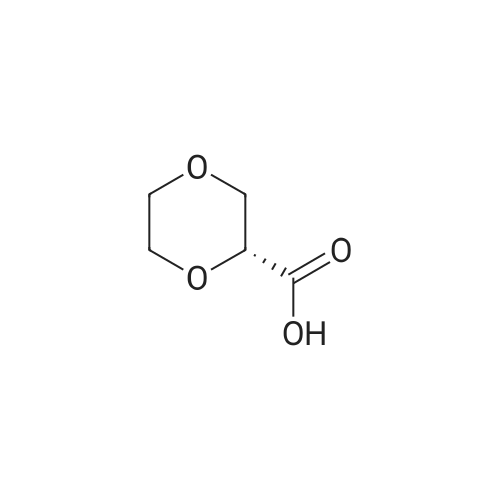
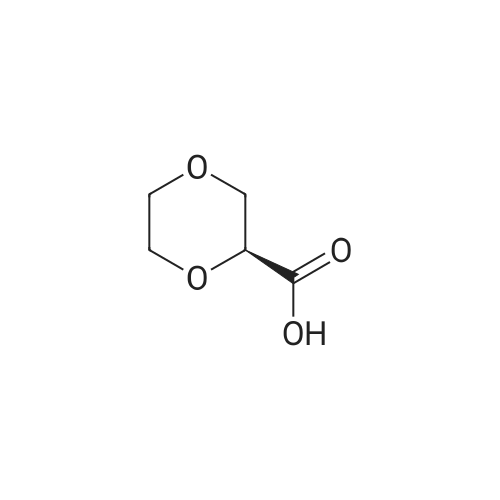
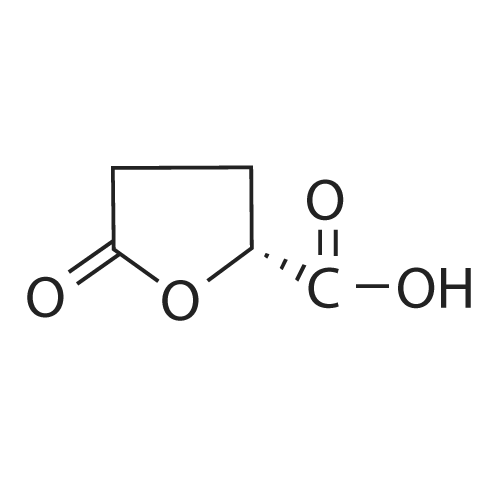
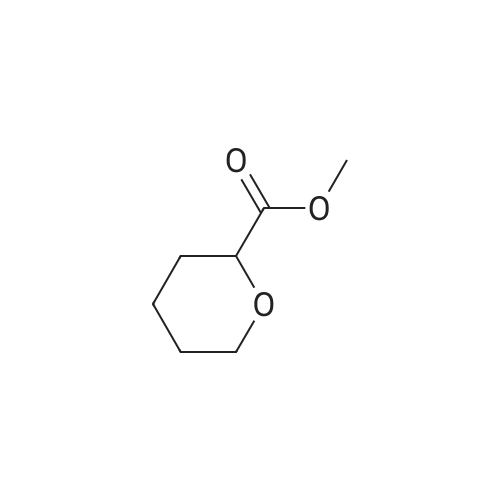
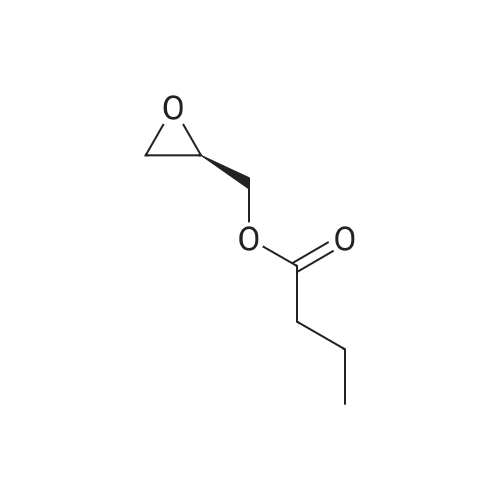
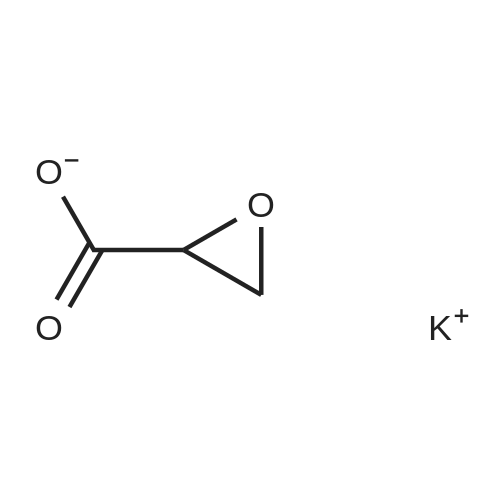
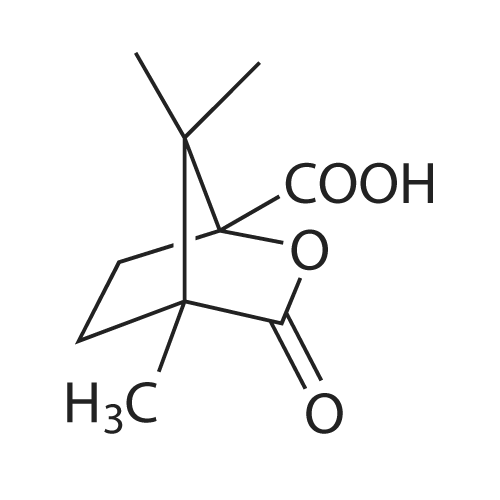
| 79% |
|
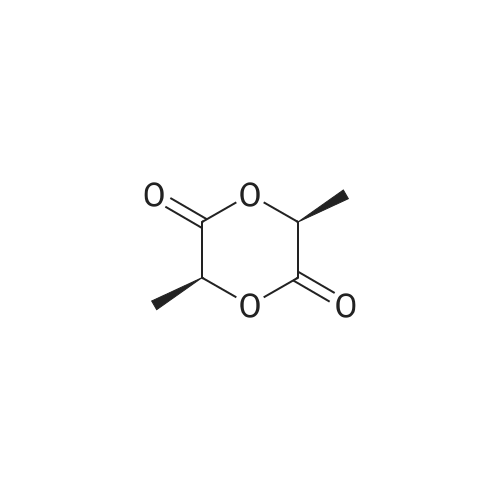
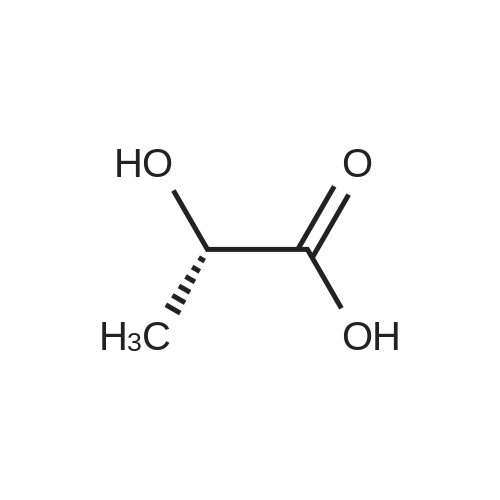
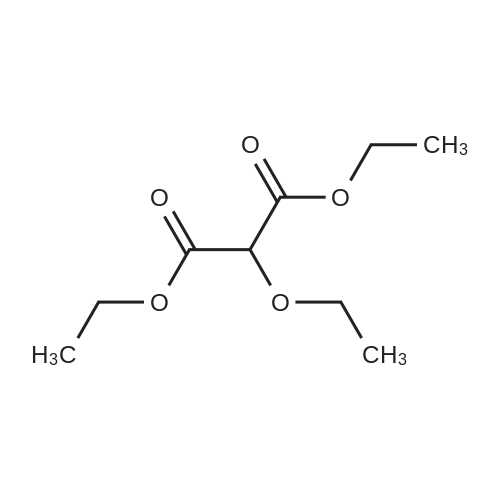
90% hydrous lactic acid (9.02 g, 90.2 mmol)Docosanol (3.27 g, 10.0 mol),3-Cl-Py-T (0.254 g, 0.90 mmol) was placed in a 50 ml round bottom flask,In a distillation apparatus equipped with a vigreux tube, at 4 kPa at 140 C.,And heated for 12 hours. The mixture was heated and stirred at 140 C. and 25 mmHg (3333 Pa) for 12 hours.All of the flask residues are carboxylate esters of lactic acid oligomer,Its weight is 9.51 g (oligomer yield 95%),The degree of polymerization was Xn = 9.It was judged that all carboxylic acid ends were protected by being 1: 2.The obtained oligomer containing catalyst was heated at 160 C. and 1 mm Hg (133.3 Pa) for 3 hours,Depolymerization was carried out by heating and depressurization to obtain 5.09 g (79%) of lactide.The purity of this crude lactide, determined from 1 H-NMR,Chemical purity> 99.5 w / w%,Mesobody contamination rate 99.99% ee.The 90% by mass L-lactic acid aqueous solution used in this example was a low-The optical purity of L lactic acid is 99% ee. Or more.The optical purity of lactic acid was measured by a known HPLC method. |
| 67.9% |
With H-BEA; In toluene; for 3h;Dean-Stark; Heating; |
In this example several zeolites were tested as catalyst for the synthesis of L-L-lactide from L-lactic acid. The following zeolites were used: CBV500, CBV600, CBV720, CBV760, and CBV780 (available from Zeolyst International, in NH4 or H-form); H-BEA (available from Sud- Chemie), NH4-ZSM-5 with various Si/AI2 ratios (available from Zeolyst International); H- MOR (available from Sud-Chemie); H-FER (available from Zeolyst International); H-MCM- 22 (available from ACSMaterial); LaX and LaY (made by starting from NaY or NaX, available from Evonik, according to C. F. Heylen and P. A. Jacobs, (Advances in Chemistry Series, 1973, 727, 490-500)). The zeolites were used in their Br0nsted acidic form (H-form). In general, when zeolites were provided (partly) exchanged with other cations (such as Sodium cations), they were exchanged and calcined to maximize the acidity and achieve the H-form. Typically, 100 mL of an aqueous solution of 0.5 M NH4CI was added per 1 .0 gram of (e.g. Na) zeolite on wet basis. The mixture was heated for 4 hours under reflux conditions. Then, the zeolite was isolated by filtration and the exchange procedure was repeated. The zeolite was isolated again, and washed with 1 L of water. In this way, the NH4-form of the zeolite is obtained. To transform this ammonium exchanged form into the Bransted acidic form, the zeolite was typically calcined for 12 hours at a temperature of 450C. A temperature ramp of 3C/min was applied. The resulting zeolites were stored at room temperature in contact with air. In a typical experiment, a reaction flask was loaded with a mixture of about 10 wt% L- lactic acid (L-LA) in toluene. Unless mentioned otherwise, the solution was prepared by mixing 1 g of 90 wt% L-LA (aqueous, obtained from Acros Organics) with 10 ml toluene. In one experiment (using the H-FER zeolite as catalyst), the solution was prepared by mixing 1 .65g of 50 wt% L-LA (aqueous, obtained from Sigma-Aldrich) with 10 ml toluene. In the conditions used for the experiments herein, no significant difference was observed between these starting solutions under these conditions. The zeolite was added to the reaction mixture (about 0.5 g of zeolite per 10 mL solution), and the mixture was heated by placing the reaction flask in a hot oil bath at a temperature of about 130C, and continuously mixed, the temperature of the reaction mixture was dependent on the used solvent and composition. A Dean-Stark trap was used for removal of water from the reaction mixture. Typically, the reaction mixture was heated for about 3 hours under stirring, after which the mixture was cooled to room temperature. The relative amounts of lactic acid oligomers, lactic acid, and lactide in the reaction mixture after 3 hours was indicative of the yield obtainable with each catalyst, as the reaction mixture typically does not change significantly after 3 hours for a good catalyst. This can be appreciated from Figure 1 , which shows the relative amount of reaction products in a reactor at different times, using a H-BEA zeolite catalyst with a Si/AI2 ratio of 25. However, it is noted that for some catalysts, the maximal concentrations may be obtained faster. Reference experiments were conducted using the known catalysts sulfuric acid (0.01 g per 10 mL solution) and Amberlyst 15 Wet (about 0.5 g per 10 mL solution). The amount of reference catalysts is chosen such that the total amount of acid sites is similar to the amount of acid sites of the zeolites, thus allowing a fair comparison. For each experiment, the total conversion rate of the lactic acid, and the lactide yield were determined via 1H NMR. Also control measurements using gas chromatography with flame ionization detector (GC/FID) and high-pressure liquid chromatography (HPLC) with uv-visible detector were performed. The total conversion of the lactic acid includes the fraction of lactic acid which had reacted to lactide, trimers, or other oligomers. The lactide yield only includes the fraction of fed lactic acid which has reacted to lactide. All zeolites having two or three interconnected and non-parallel channel systems, with at least one of said systems comprising 10-or more-membered ring channels and a framework Si/AI2 ratio of at least 24, and all zeolites having three interconnected and non- parallel channel systems, with at least two of said channel systems comprising 10-or more-membered ring channels and a framework Si/AI2 ratio of at least 6, provided lactide yields above 20%, up to about 70%. The results of the various experiments are summarized in Table 1. It is noted that for some zeolites, the framework Si/AI2 ratio, may differ from the bulk Si/AI2 ratio. For all zeolites, the framework Si/AI2 ratio is provided, as this is most relevant ratio for the catalysis. For some zeolites, the bulk Si/AI2 ratio is also provided (between brackets). Table 1 Number of Si/AI2 LA Lactide Catalyst interconnected Topology Ring size framework conversion yield Name non parallel rat... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping