|
With triethylamine;dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; dichloro bis(acetonitrile) palladium(II); at 110℃; for 4.0h;Inert atmosphere; |
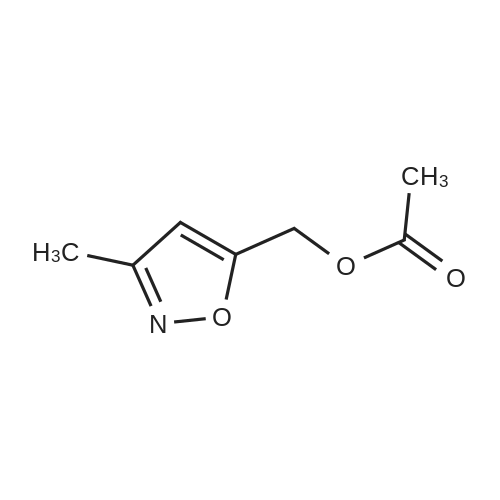
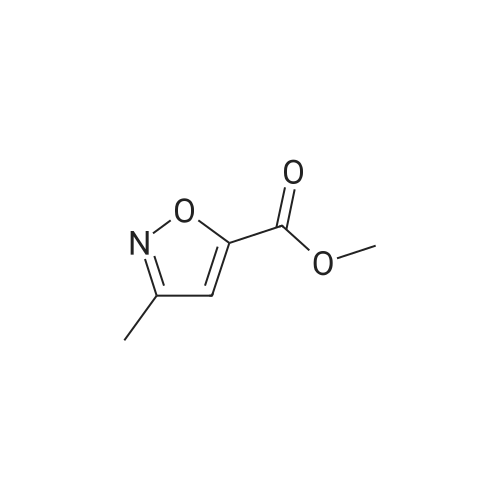
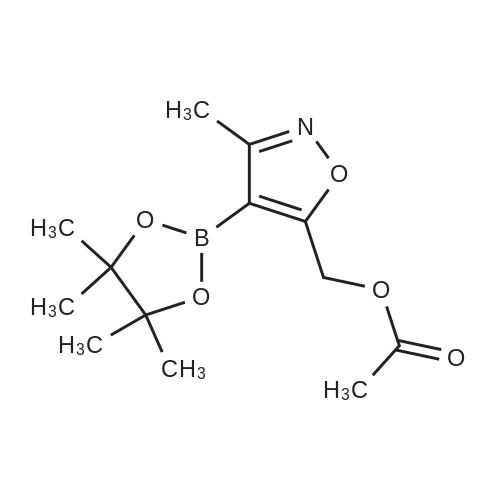
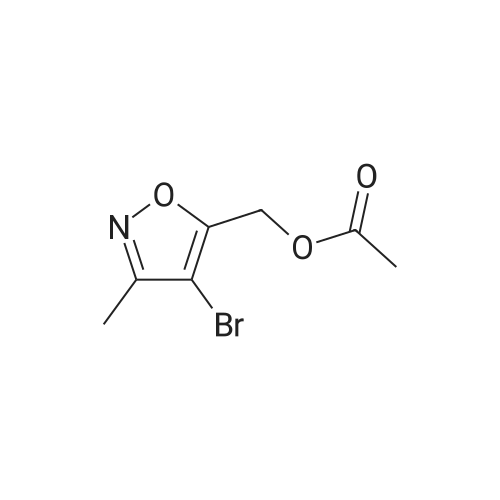
[00238] -Methyl-4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)isoxazol-5-yl)methylacetate. To a 500 mL flask (under N2 (g)) was addeddichlorobis(acetonitrile)palladium(II) (0.551 g, 2.12 mmol) and dicyclohexyl(2',6'- dimethoxybiphenyl-2-yl)phosphine (3.50 g, 8.53 mmol). To the solids were sequentially added a solution of <strong>[1380089-33-7](4-bromo-3-methylisoxazol-5-yl)methyl acetate</strong> (24.8 g, 106 mmol) in 1,4-dioxane (65 mL), Et3N (44.3 mL, 318 mmol), and 4,4,5,5-tetramethyl- 1,3,2- dioxaborolane (24 mL, 160 mmol). The flask was sequentially evacuated and purged under N2, and this process was repeated three times. The reaction mixture was heated to 110 C (under a constant stream of N2 (g)) and allowed to stir for -4 h. LC-MS analysis at this point showed complete conversion of the starting bromo-isoxazole. The reaction mixture was cooled to room temperature and EtOAc (100 mL) was added. After 15 min of stirring, the suspension was filtered over a pad of Celite. The filter cake was washed with EtOAc (3 x 100 mL), concentrated in vacuo, and the solvent was switched using 1,4-Dioxane (2 x 50 mL). The borate ester with used without further purification. |
|
With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; dichloro bis(acetonitrile) palladium(II); triethylamine; In 1,4-dioxane; at 110℃; for 4.0h;Inert atmosphere; |
To a 500 mL flask (under N2 (g)) was added dichlorobis(acetonitrile)palladium(II) (0.55 1 g, 2.12 mmol) and dicyclohexyl(2?,6?- dimethoxybiphenyl-2-yl)phosphine (3.50 g, 8.53 mmol). To the solids were sequentially added a solution of <strong>[1380089-33-7](4-bromo-3-methylisoxazol-5-yl)methyl acetate</strong> (24.8 g, 106 mmol) in 1,4-dioxane (65 mL), Et3N (44.3 mL, 318 mmol), and 4,4,5,5-tetramethyl-1,3,2- dioxaborolane (24.0 mL, 160 mmol). The flask was sequentially evacuated and purged under N2, and this process was repeated three times. The reaction mixture was heated to 110 C (under a constant stream of N2 (g)) and allowed to stir for -4 h. LC-MS analysis at this point showed complete conversion of the starting bromo-isoxazole. The reaction mixture was cooled to room temperature and EtOAc (100 mL) was added. After 15 mm of stirring, the suspension was filtered over a pad of Celite. The filter cake was washed with EtOAc (3 x 100 mL), concentrated in vacuo, and the solvent was switched using 1,4-Dioxane (2 x 50 mL). The borate ester with used without further purification. |
| 12 g |
With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; dichloro bis(acetonitrile) palladium(II); triethylamine; In 1,4-dioxane; at 110℃; for 16.3333h;Inert atmosphere; |
To a 200 mL flask (under N2) was added dichlorobis(acetonitrile)palladium(II)(0.22 g, 0.85 mmol) and dicyclohexyl(2?,6?-dimethoxybiphenyl-2-yl)phosphine (1.40 g, 3.41 mmol). To the solid were sequentially added a solution of(4-bromo-3-methyl- isoxazol-5y1)methyl acetate (10.0 g, 42.73 mmol) in dry 1,4-dioxane (100 mL), dry Et3N(17.82 mL, 128.20 mmol), and 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (15.50 mL,106.83 mmol). The flask was sequentially evacuated, purged under N2, and repeated for20 mm. The mixture was heated to 110 C (under N2) for 16 h. The mixture was cooledto RT and filtered through celite pad followed by washing with EtOAc. The filtrate wasconcentrated under reduced pressure. The residue was used for further step withoutfurther purification (12.0 g). ?H NMR (400 MHz, CDC13): oe 5.31 (s, 2H), 2.37 (s, 3H),2.11 (s, 12H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping