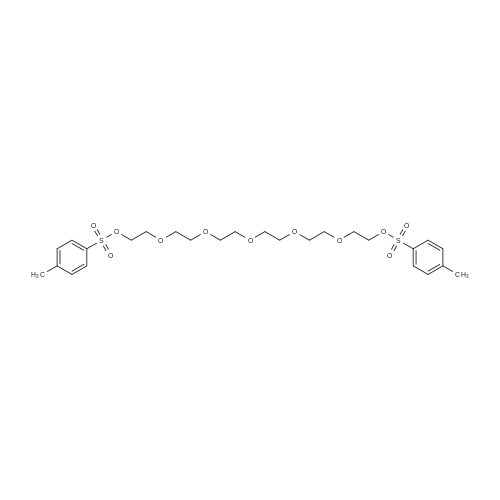
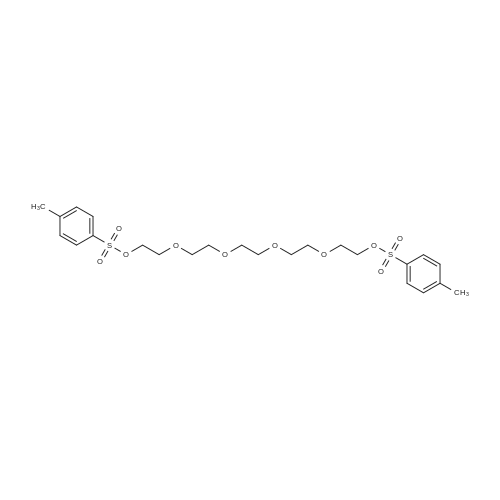
| 89.5% |
|
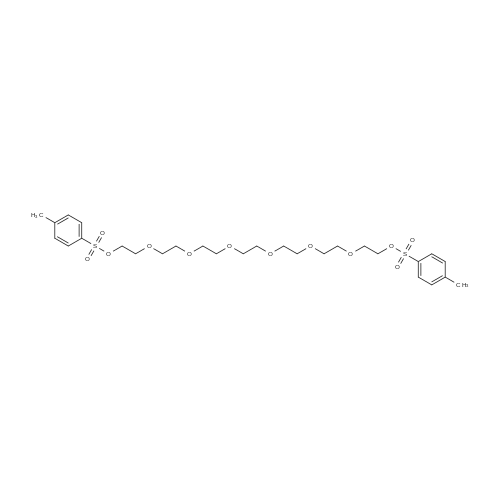
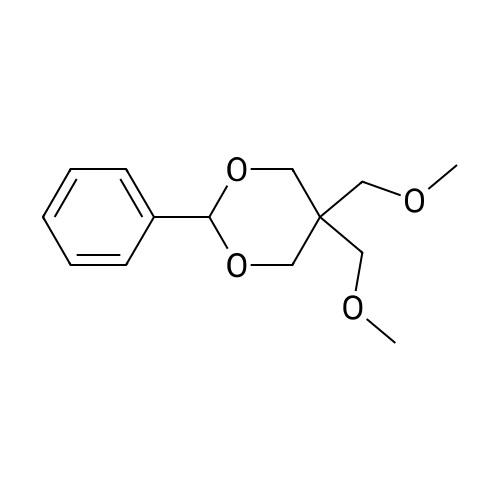
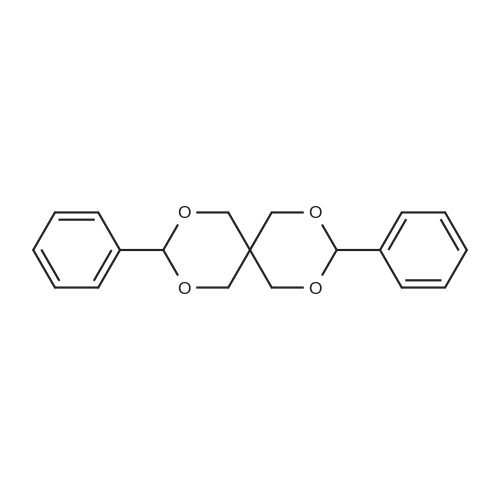
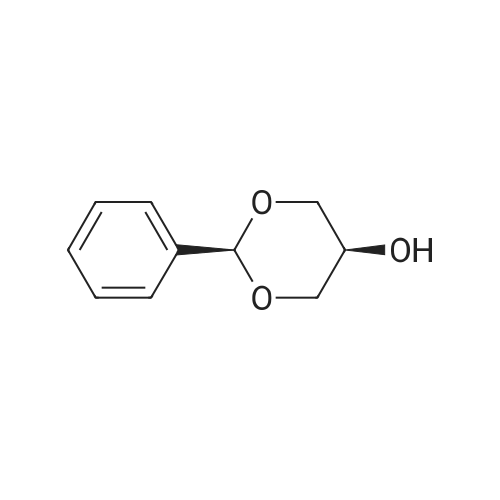
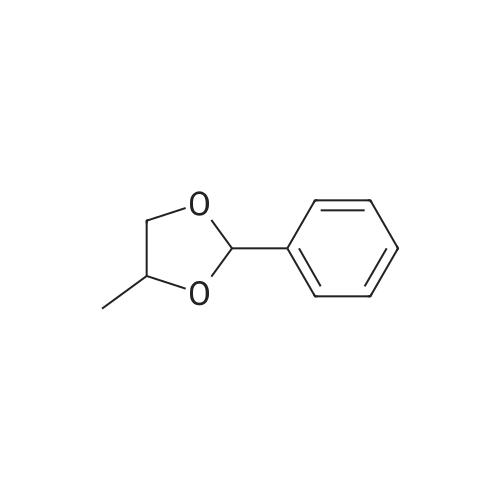
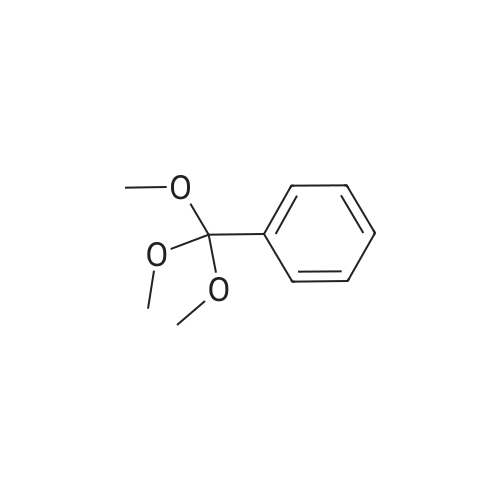
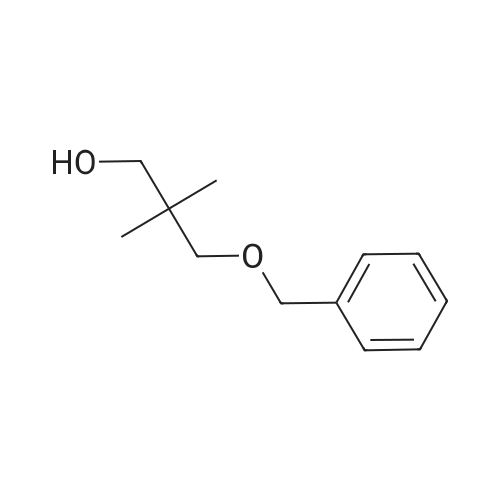
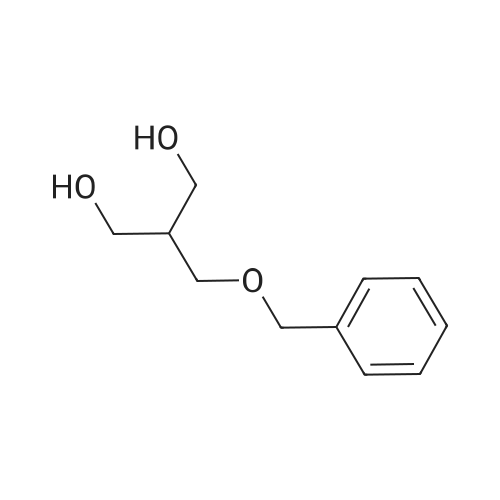
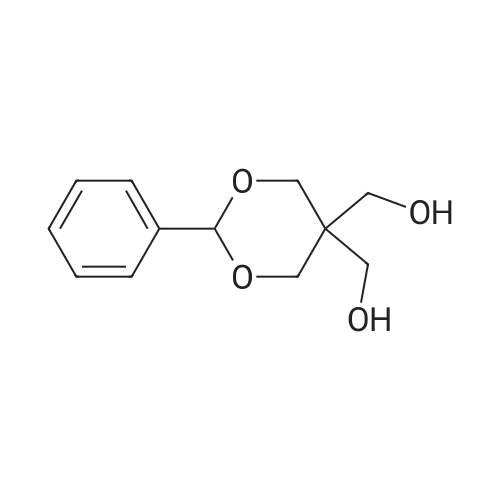
To a suspension of NaH (13.2 g, 60% in mineral oil, 230.0 mmol) in THF (40 ml) under argon at0 oc was added a solution of diol (5109, 4.92 g, 22.0 mmol) in THF (20 ml) dropwise; the resultingmixture was warmed to room temperature and stirred for 1 h. The reaction mixture was cooled to 0 C, a10 solution of propargyl bromide (18.6 g, 158.4 mmol) in THF (25 ml) was added slowly, and the resultingmixture was warmed to room temperature and stirred overnight at 40 C. After the product wasconsumed, as observed by TLC, the reaction was quenched by dropwise addition of water at 0 C, andthe resulting mixture was extracted with dichloromethane (50 ml x 2). The combined organic layers werewashed with brine and dried over anhydrous Na2S04, filtered, and evaporated to give a residue, which15 was purified by flash silica gel column using ISCO companion (hexane/ethyl acetate, 0- 30%) to give5.92 g (89.5%) of compound S110 as an oil. 1H NMR (500 MHz, CDCI3;ppm): delta7.49-7.47 (dd, J8.0, 1.5Hz, 2H), 7.38-7.34 (m, 3H), 5.43 (s, 1 H), 4.21 (d, J 2.5 Hz, 2H), 4.12 (t, J 2.5 Hz, 4H), 4.10 (s, 1 H), 3.91(s, 1 H), 3.89 (s, 1 H), 3.37 (s, 2H); ESI MS for C15H20O4calculated 300.34, observed [M+H]+ 301.3 |
| 89.5% |
|
To a suspension of NaH (13.2 g, 60% in mineral oil, 230.0 mmol) in THF (40 mE) under argon at 00 C. was added a solution of diol (S109, 4.92 g, 22.0 mmol) in THF (20 mE) dropwise; the resulting mixture was warmed to room temperature and stirred for 1 h. The reaction mixture was cooled to 0 C., a solution of propargyl bromide (18.6 g, 158.4 mmol) in THF (25 mE) was added slowly, and the resulting mixture was warmed to room temperature and stirred overnight at 40 C. After the product was consumed, as observed by TEC, the reaction was quenched by dropwise addition of water at 0 C., and the resulting mixture was extracted with dichloromethane (50 mEx2). The combined organic layers were washed with brine and dried over anhydrous Na2504, filtered, and evaporated to give a residue, which was purified by flash silica gel column using ISCO companion (hexane/ethyl acetate, 0-30%) to give 5.92 g (89.5%) of compound 5110 as an oil. ?H NMR (500 MHz, CDC13, ppm): oe7.49-7.47 (dd, J 8.0, 1.5 Hz, 2H), 7.38-7.34 (m, 3H), 5.43 (s, 1H), 4.21 (d, J 2.5 Hz, 2H), 4.12 (t, J 2.5 Hz, 4H), 4.10 (s, 1H), 3.91 (s, 1H), 3.89 (s, 1H), 3.37 (s, 2H); ESI MS for C,8H2Q04 calculated 300.34, observed [M+H]301.3. |
| 89.5% |
|
under argon at 0 oC was added a solution of diol (S109, 4.92 g, 22.0 mmol) in THF (20 mL) dropwise; the resulting mixture was warmed to room temperature and stirred for 1h. The reaction mixture was cooled to 0 oC, a solution of propargyl bromide (18.6 g, 158.4 mmol) in THF (25 mL) was added slowly, and the resulting mixture was warmed to room temperature and stirred overnight at 40 oC. After the product was consumed, as observed by TLC, the reaction was quenched by dropwise addition of water at 0 oC, and the resulting mixture was extracted with dichloromethane (50 mL x 2). The combined organic layers were washed with brine and dried over anhydrous Na2SO4, filtered, and evaporated to give a residue, which was purified by flash silica gel column using ISCO companion (hexane/ethyl acetate, 0 - 30%) to give 5.92 g (89.5%) of compound S110 as an oil.1H NMR (500 MHz, CDCl3; ppm): d7.49-7.47 (dd, J 8.0, 1.5 Hz, 2H), 7.38-7.34 (m, 3H), 5.43 (s, 1H), 4.21 (d, J 2.5 Hz, 2H), 4.12 (t, J 2.5 Hz, 4H), 4.10 (s, 1H), 3.91 (s, 1H), 3.89 (s, 1H), 3.37 (s, 2H); ESI MS for C18H20O4 calculated 300.34, observed [M+H]+ 301.3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping