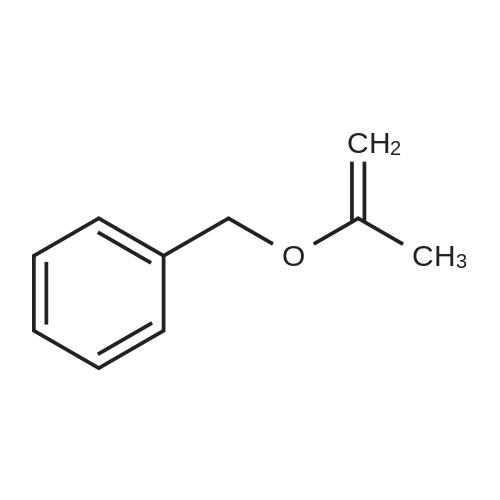
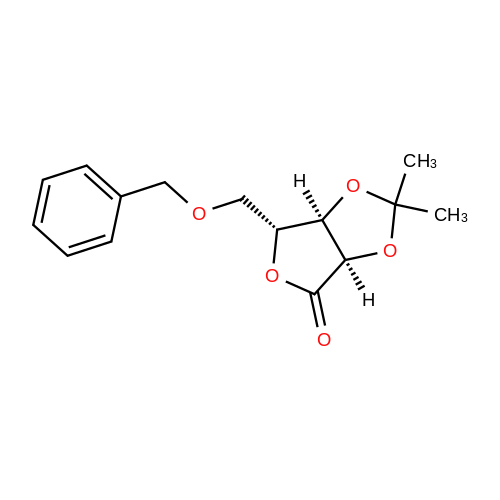
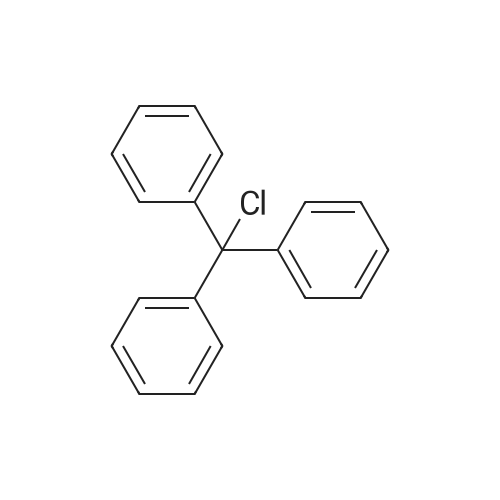
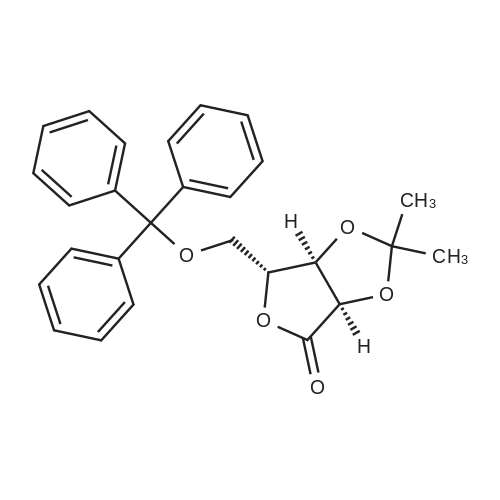
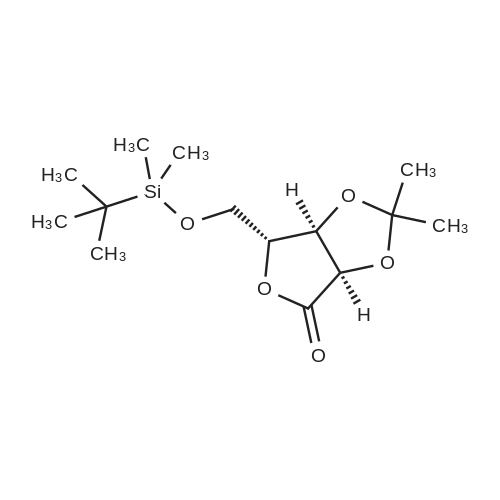
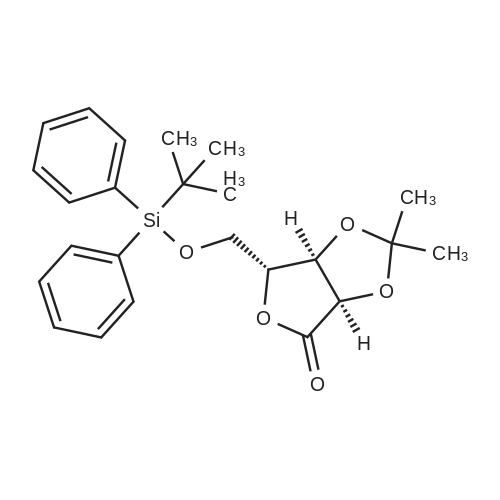
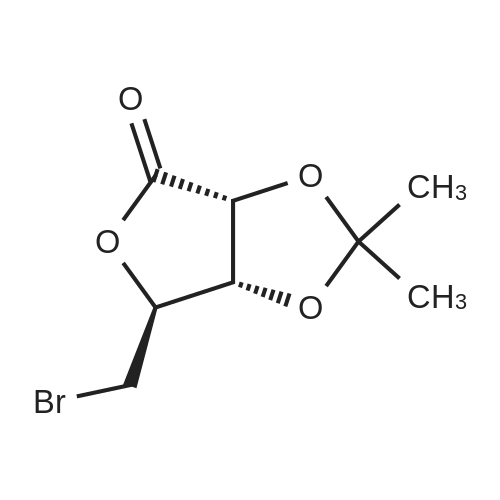
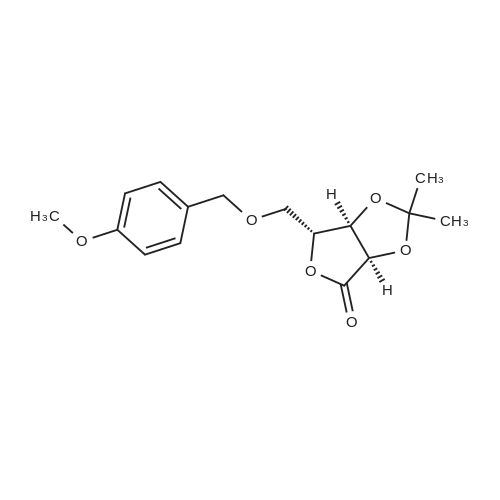
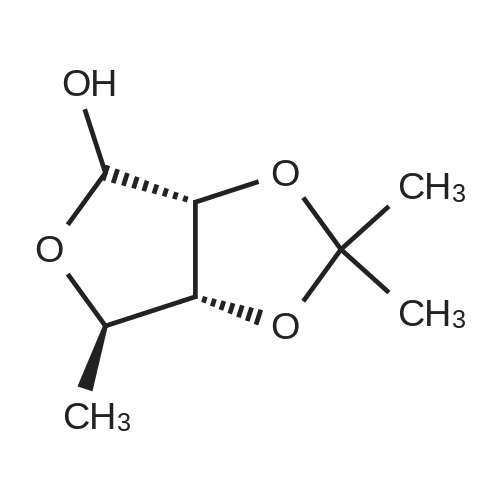
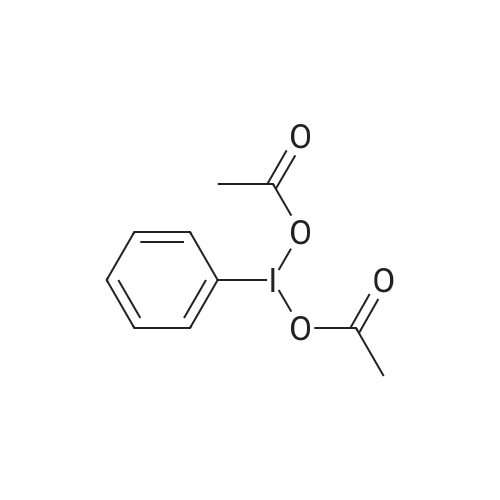
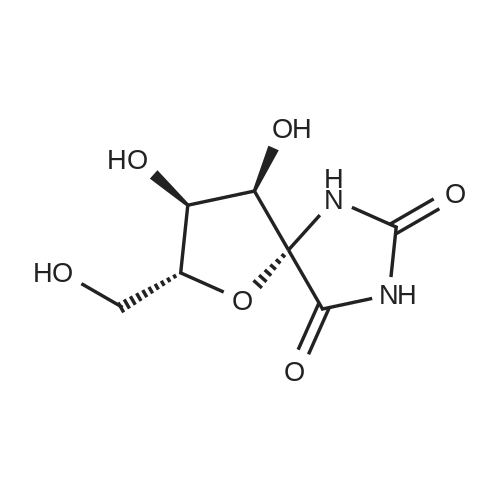
| 98% |
With copper(II) sulfate for 72h; Ambient temperature; |
|
| 98% |
With toluene-4-sulfonic acid for 16h; |
10
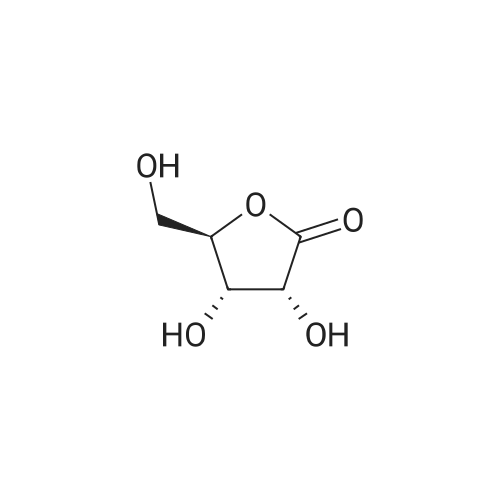
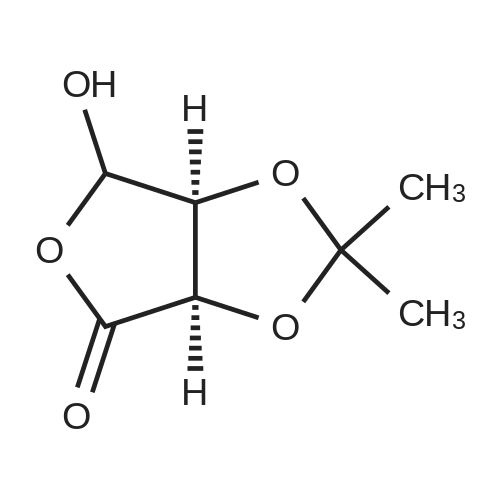
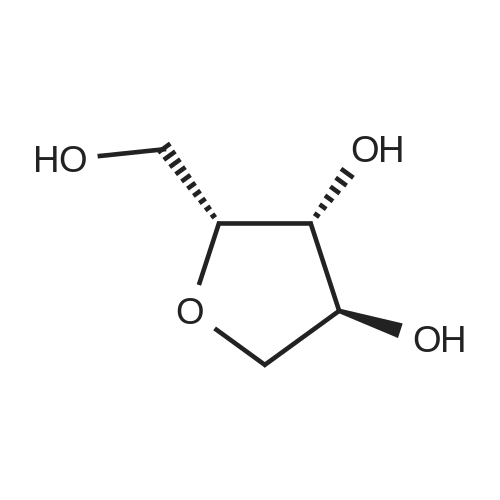
A mixture of (3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)dihydrofuran-2(3H)-one (40 g, 270 mmol) and p-toluenesulfonic acid monohydrate (500 mg, 2.63 mmol) was stirred in acetone (volume: 600 ml) for 16h. Then solid NaHCC (5g) was added, and the reaction mixture was stirred for lhour. The reaction mixture was filtered through celite and concentrated under reduced pressure to dryness. The product was triturated with hexanes, and the solid was collected by filtration and dried to give a 98% yield. |
| 95% |
With hydrogen cation for 24h; Ambient temperature; |
|
| 94% |
With sulfuric acid for 6h; Ambient temperature; |
|
| 93% |
With sulfuric acid at 20℃; for 72h; |
Synthesis of 2,3-O-isopropylidene-D-ribonic acid-1,4-lactone
A solution of D-ribonic acid-l,4-lactone (270.0 g, 1.823 mol) and sulphuric acid (18.0 g, 0.182 mol, 0.1 equiv.) in acetone (2.79 L) was stirred at room temperature for 3 days. The reaction mixture was quenched by the addition of solid sodium bicarbonate (-450 g), filtered and the filtrate evaporated. The residue was partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate; the combined organic layers were dried over magnesium sulphate, filtered and concentrated under reduced pressure to give the desired product as a white solid (318.8 g, 93%). 1H NMR (300 MHz, CDC13) δ 4.83 (d, J= 5.5 Hz, 1H), 4.77 (d, J= 5.5 Hz, 1H), 4.64-4.62 (m, 1H), 3.99 (ddd, J= 2.3, 5.5 and 12.4 Hz, 1H), 3.81 (ddd, J= 2.3, 5.5 and 12.4 Hz, 1H), 2.67 (t, J= 5.5 Hz, 1H), 1.46 (s, 3H), 1.37 (s, 3H). |
| 92% |
With iodine for 12h; Ambient temperature; |
|
| 91% |
With sulfuric acid for 5h; |
|
| 90% |
With sulfuric acid at 0 - 20℃; for 5h; |
|
| 89% |
With sulfuric acid at 20℃; |
|
| 86% |
With sulfuric acid at 0 - 20℃; |
|
| 85% |
for 20h; Heating; |
|
| 85% |
With sulfuric acid for 5h; Ambient temperature; |
|
| 85% |
With sulfuric acid at 20℃; for 12h; |
|
| 84% |
With sulfuric acid |
|
| 81% |
With hydrogenchloride for 18h; Ambient temperature; |
|
| 80% |
With sulfuric acid at 20℃; for 12h; |
|
| 80% |
With sulfuric acid In acetone at 0 - 20℃; for 12h; |
3
The expoxide reagent may be synthesized as shown in Scheme 3. D-Ribonolactone 12 was converted to the 2,3-O-isopropylidene-D-ribonolactone 13; To a suspension of D-ribonolactone 12 (10 g, 68 mmol) in actone (200 mL) was added concentrated sulfuric acid (4 mL) dropwise while the solution was cooled in an ice bath. The starting material dissolved in 5 minutes. The mixture was stirred for 12 h at room temperature. Ammonia gas was passed through the ice-cooled solution. The resulting white solid was filtered off and the filtrate was concentrated under reduced pressure. The crude product was purified by column chromatography (Hexanes-EtOAc, 1:3) to afford 13 (10.6 g, 80%) as a white solid: mp 134-137° C.; lit. mp 135-138. |
| 79% |
With hydrogenchloride at 26℃; for 18h; |
|
| 75% |
With hydrogenchloride In water at 20℃; for 18h; |
|
| 62% |
With hydrogenchloride for 4h; Ambient temperature; |
|
| 48% |
Stage #1: acetone; D-Ribono-1,4-lactone at 20℃; for 1h; Large scale;
Stage #2: With sulfuric acid at 10℃; Large scale; |
1.2 Step 2: Preparation of 2,3-O-isopropylidene D-Ribono-1,4-lactone (19c)
A 50-L jacketed reaction vessel was charged with D-ribono-1,4-lactone (19b) (3.0 kg, 20.27 mol), and 30 L of ACS grade acetone. The reaction mixture was stirred at roomtemperature for 1 h. The internal temperature of the reaction vessel was lowered to 10 ocand cone. sulfuric acid (49 mL) was added slowly to the reaction mixture. Upon addition of the sulfuric acid the internal reaction temperature was allowed to warm up slowly. Thereaction mixture was stirred at this temperature for 2.5- 3 h. The reaction was monitoredby TLC (TLC; 9:1, methylenechloride:methyl alcohol, R1= 0.75). The reaction mixturewas neutralized by addition of solid sodium bicarbonate ( ~500 g) until the pH was neutral. The reaction mixture was filtered over a funnel. The solid residue containing inorganicsalts was washed with acetone (3 L). The filtrate was transferred to a 20-L evaporationflask and evaporated to dryness (50 °C, 10 mmHg) to give a semi-solid compound. Theresidue was taken in ethyl acetate (3 L) and stirred at room temperature for 4 h on rotaryevaporator. The solid 2,3-0-isopropylidene D-Ribono-1,4-lactone (19c) was collected by filtration and dried in a vacuum oven for 16 hat 40 oc (0.1 mm Hg). Yield: 1.819 kg (48%); MP 136-140 oc; 1H NMR (CDCh) 8 4.8 (dd, 2 H), 4.6 (s, 1 H), 3.85 (dd, 2 H), 1.5 (s,3 H), 1.4 (s, 3 H). |
| 48% |
With sulfuric acid at 10℃; Large scale; |
1.2 Step 2: Preparation of 2,3-O-isopropylidene D-Ribono- 1,4-lactone (lc)
Step 2: Preparation of 2,3-O-isopropylidene D-Ribono- 1,4-lactone (lc) A 50-L jacketed reaction vessel was charged with D-ribono- 1,4-lactone (lb) (3.0 kg, 20.27 mol), and 30 L of ACS grade acetone. The reaction mixture was stirred at room temperature for 1 h. The internal temperature of the reaction vessel was lowered to 10 °C and cone, sulfuric acid (49 mL) was added slowly to the reaction mixture. Upon addition of the sulfuric acid the internal reaction temperature was allowed to warm up slowly. The reaction mixture was stirred at this temperature for 2.5 - 3 h. The reaction was monitored by TLC (TLC; 9: 1, methylenechloride: methyl alcohol, R/ = 0.75). The reaction mixture was neutralized by addition of solid sodium bicarbonate (-500 g) till the pH was neutral. The reaction mixture was filtered over a funnel. The solid residue containing inorganic salts was washed with acetone (3 L). The filtrate was transferred to a 20-L evaporation flask and evaporated to dryness (50 °C, 10 mm Hg) to give a semi-solid compound. The residue was taken in ethyl acetate (3 L) and stirred at room temperature for 4 h on rotary evaporator. The solid 2,3-O- isopropylidene D-Ribono-l,4-lactone (lc), was collected by filtration and dried in a vacuum oven for 16 h at 40 °C (0.1 mm Hg). Yield: 1.819 kg (48%); MP 136 -140 °C; 1H NMR (CDCls) δ 4.8 (dd, 2 H), 4.6 (s, 1 H), 3.85 (dd, 2 H), 1.5 (s, 3 H), 1.4 (s, 3 H). |
| 42% |
With hydrogenchloride at 25℃; for 0.25h; |
Preparation of 2,3-O-isopropylidene-D-ribonolactone (5)3,4,7
D-Ribonolactone 2 (148 mg, 1.0 mmol) and acetone (1.5 mL) were added into a 10 mL round-bottomed flask with a magnetic stirring bar and then 0.1 mL (2 drops) of 12 mol L-1 HCl was added at 25 °C. After 15 min, 0.5 mLof CH2Cl2 was poured to dissolve the white solid formed during the course of the reaction. Then the white mixture was quenched by pouring 300 mg of powdered NaHCO3 in small portions (5 min, until CO2 effervescence ceases), which turned the mixture into a yellow color. The liquid phase was decanted and a second portion of CH2Cl2 (0.5 mL) was added to the reaction residue to extract additional white solid. This process was repeated a second time and the combined CH2Cl2 extracts were evaporated to dryness using a rotary evaporator to give 5 as a colorless solid (80 mg, 42% yield). |
|
With hydrogen cation |
|
|
With sulfuric acid |
|
|
With sulfuric acid for 5h; Ambient temperature; |
|
|
With sulfuric acid at 20℃; for 30h; |
1,4-Anhydro-5-O-tert-butyldiphenylsilyl-2,3-O-isopropylidene-4-seleno-D-ribitol (3)21,23
To asolution of S1 in acetone (500 mL) was added conc. H2SO4 (2.5 mL), and the reaction mixture was stirred for 30 h at room temperature. After being cooled to 0 °C, the reaction mixture was neutralized with sodium bicarbonate. The resulting solids were filtered through a Celite pad, and washed with hot acetone. The combined filtrate and washings were concentrated in vacuo, and partitioned between CHCl3 and H2O, the organic layer was washed with brine, dried (Na2SO4) and concentratedin vacuo to give crude S2 (45.32 g). |
|
With sulfuric acid at 20 - 30℃; for 12h; Inert atmosphere; |
The brown solid was dissolved in 500 L of acetone (1185 kg, 20403 mol, 41 equiiv) and 7.5 L of sulfuric acid (Sulfuric acid) was heated to a boiling point under nitrogen condition for 4 hours. Thereafter, the reaction mixture was cooled to 20-30 ° C. and kept at temperature for 8 hours. the pH of the reaction mixture was adjusted to 5.5-6.0 using sodium hydroxide, filtered, and titrated in a vacuum at 40 ° C. to obtain a solid product. the crude solid product was dissolved by stirring at 250 ° C. with 250 L of ethyl acetate, then the solution was filtered and titrated in vacuo by 50% of the initial volume. the solution was then cooled to -5 ° C to give solid compound 9, which was purified by filtration and drying in 35 ° C vacuum. |
|
With sulfuric acid |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping