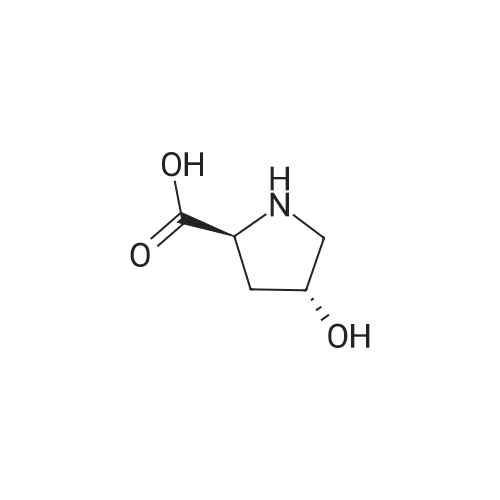
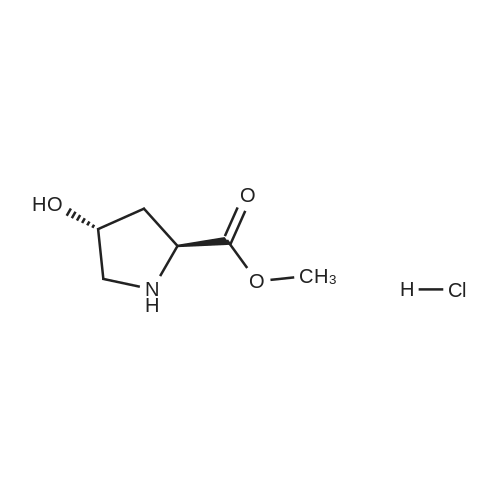
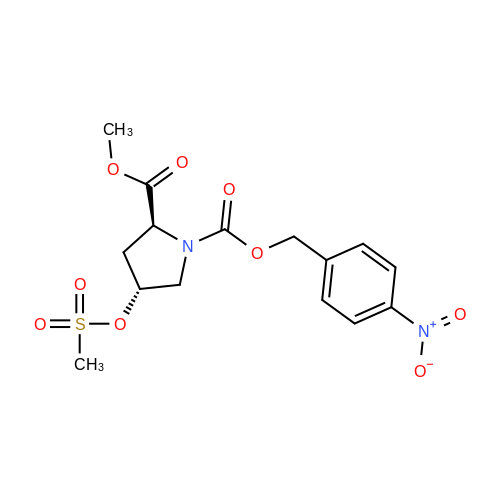
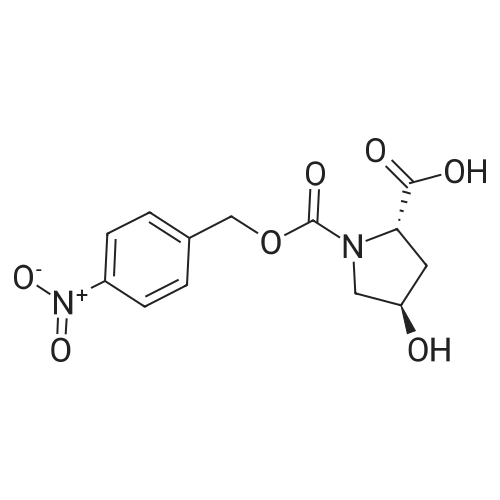
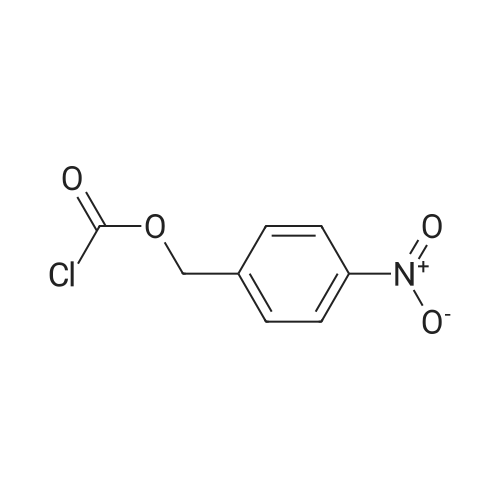
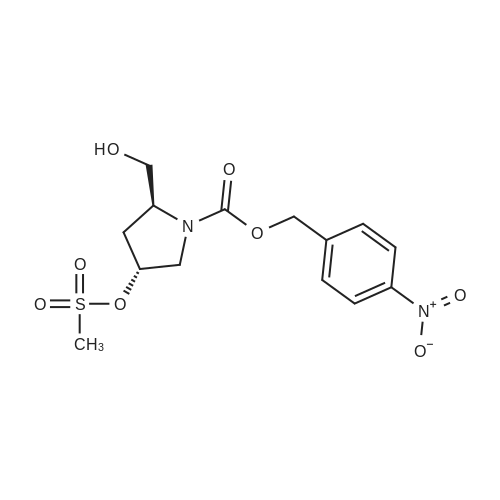
|
With trichlorophosphate In methanol Reflux; |
a
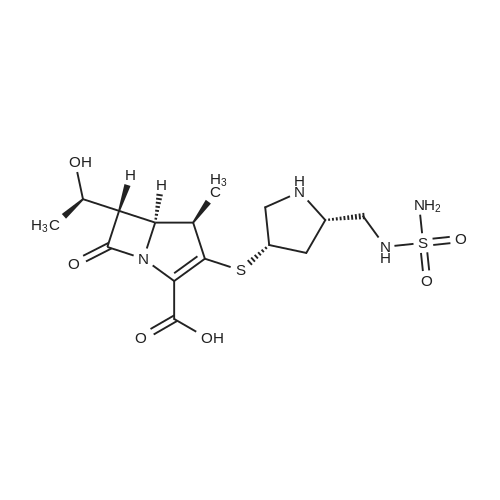
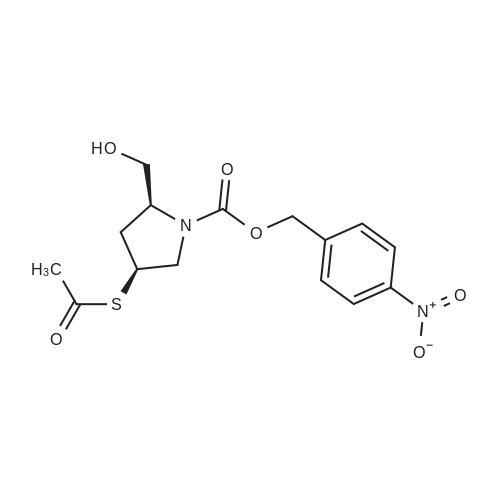
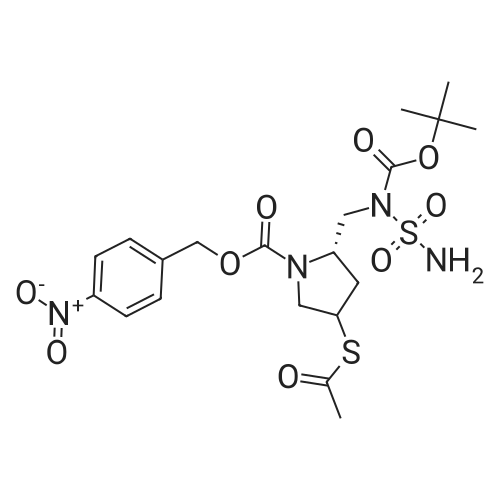
Example-a: To a slurry of (2S,4S)-4-Acetylthio-2-(N-sulfamoyl-tert- butoxycarbonylaminomethyl)-l-(4-nitrobenzyloxycarbonyl)pyrrolidine (5 g) in methanol (30 mL) was added phosphorous oxy chloride (2 g). The resultant mixture was heated to reflux till completion of reaction. To the cooled reaction mixture were added ethyl acetate (25 mL), purified water (10 mL) and saturated sodium chloride solution (15 mL) were added and separated the organic layer. The aqueous layer was extracted with ethyl acetate and the combined organic layer was washed successively with purified water, saturated sodium bicarbonate solution and saturated sodium chloride solution. Solvent was removed and to the residue was added toluene (20 mL). The solid formed was filtered and washed with diisopropyl ether. Drying the product afforded pure crystalline title compound. |
|
With triphenylphosphine; trichlorophosphate In methanol at 25 - 67℃; |
1
To a slurry of (2S,4S)-4-Acetylthio-2-(N-sulfamoyl-tert-butoxycarbonyl aminomethyl)- l-(4-nitrobenzyloxycarbonyl)p rrolidine (50g) and triphenyl phosphine (2.5 g) in methanol (150 ml), phosphorous oxychloride (3.8 g) was added at 25-30°C. The resultant mixture was heated to 63-67°C and maintained for 4-8 hours and cooled to 25-30°C. To the reaction mixture ethyl acetate (250 ml), purified water and saturated sodium chloride solution were added and the layers were separated. The organic layer was washed with saturated sodium chloride solution and concentrated to get a thick mass which was dissolved in tetrahydrofuran (50 ml). The tetrahydrofuran layer containing the product was charged slowly into purified water containing diisopropyl ether. The resultant mass was stirred and cooled. The solid product formed was filtered and washed with purified water. The wet material was suck dried under vacuum and unloaded. The wet product was stirred with toluene (100 ml) at 25-30°C and filtered. The product was washed with toluene (50 ml) and dried under vacuum.Yield: 31.2 g Purity by HP LC: 99.1% |
|
With methanol; sulfuric acid at 65℃; for 2.5h; Large scale; |
|
|
With n-valeryl chloride In methanol at 48 - 50℃; for 18h; |
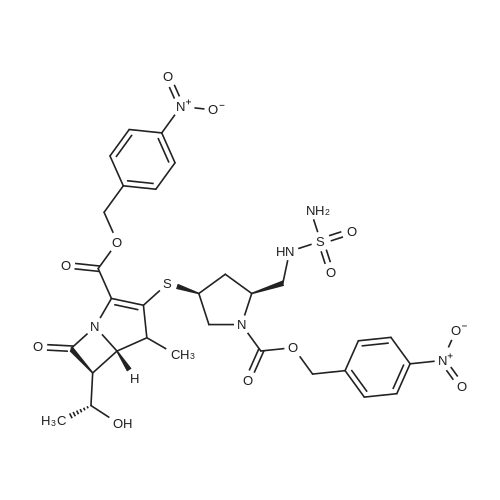
A mixture of (2S, 4S)-4-(acetylthio)-2-(((tert-butoxycarbonyl)(sulfamoyl)amino) methyl)pyrrolidine-1-carboxylic 4-nitrobenzoic anhydride (1b,17.1kg, 32.1mol) and valerylchloride (1.7 kg, 14.09 mol) in methanol (75 L) was heated to 50C and stirred at 48-50C for 18 h. After completion of reaction, the reaction mixture was diluted withdichloromethane (150 L) and water (150 L). The separated organic layer was washed withdemineralised water (150 L), followed by 1% w/v aqueous NaCl solution (150 L). Afterwashings the organic layer was concentrated to oil which was dissolved in N, N-dimethylformamide(30 L). The solution was added to (4R,5R,6S)-4-nitrobenzyl3-((diphenoxyphosphoryl)oxy)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]-hept-2-ene-2-carboxylate(3b, 15kg, 25.23 mol) in N,N-dimethylformamide (45 L) at -40C. Later N-ethyldiisopropylamine (4.53 kg, 35.05 mol) was added to reaction mass and stirred for 4 h at40C. The temperature of reaction was then raised to 20C and stirred for 6 h. Thereaction mixture was diluted with ethylacetate (150 L), water (90 L) and acidified topH 2.8 with 1N aqueous hydrochloric acid (12.45 L) at 5C. The two layers were separated,and the aqueous layer extracted with ethyl acetate (90 L). The combined organiclayers washed with 0.25% w/v aqueous NaCl solution (2£150 L) at 15C and concentratedto a sticky mass which was dissolved in tetrahydrofuran (195 L) at 20C. A buffersolution (pH 7.1), prepared by mixing N-methylmorpoline (2.55 kg, 25.21 mol), aceticacid (1.095 kg, 18.25 mol) and water (82.5 L) at 20C, which was added to the abovereaction mass. The solution was added to pre-reduced Pd/C, obtained by stirring 10% w/wPd/C (22.5 kg, 50% w/w wet) in DM water (82.5 L) for 1 h under hydrogen atmosphere(0.3 Mpa) at 20C. The reaction mixture was stirred for 2.5 h under hydrogen atmosphere(0.8 Mpa) at 20C. The reaction mass was filtered through a Celite bed and the filtrate waswashed with a mixture of water (15 L) and tetrahydrofuran (15 L). The aqueous filtrateobtained was extracted with ethyl acetate (210 L) at 20C. After addition of seed crystalsof doripenem (0.03 Kg) at 18C and slow addition of of isopropanol (507 L) over a periodof 5 h, the slurry mass was cooled to 0-5C and stirred for 8 h. The slurried mass wasfiltered at 0-5C to give the wet product which was washed with 20% v/v aqueous isopropanol(30 L, 0-5C) and dried under vacuum at 45-50C for 8 h to give doripenem hydrate5 (7.89 kg with 71% yield and 99.68% a/a HPLC purity). |
| 27 kg |
With sulfuric acid In methanol at 62 - 65℃; Inert atmosphere; Large scale; |
1.1 Step 1: Side chain deprotection
In a 100L reaction vessel, 33.6kg of anhydrous methanol and 3.84kg concentrated sulfuric acid were slowly added while stirring under nitrogen. 8.16kg of doripenem side chain was added and is heated to 62-65°C at reflux while stirring. TLC (chloroform:methanol=10:1) was used to know if the reaction was complete. The reaction ended in 4-5 hours. Heating was stopped. After appropriate cooling, 45°C approximately half of the solvent was distilled off under reduced pressure, the residual solution of about 19kg; The residual solution was transferred into water containing 60kg and 54kg of ethyl acetate mixed solvent extraction tank 200L the mixture was stirred, layers were separated. The organic layer was 5% NaCl solution was washed three times with 40kg × 3; the organic layer was separated, added to 0.8kg of activated carbon and dried over anhydrous sodium 5 ~ 6kg (about 10% of the amount of solution, hereinafter the same) and dried, stirred 0.5h, filtered; and the filtrate 100L into the reaction vessel, 45 out most of the organic solvent concentrated under reduced pressure, the residue solution 25-27kg direct investment in the next reaction. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping