|
With triethylamine In propan-1-ol at 70℃; for 8h; |
88
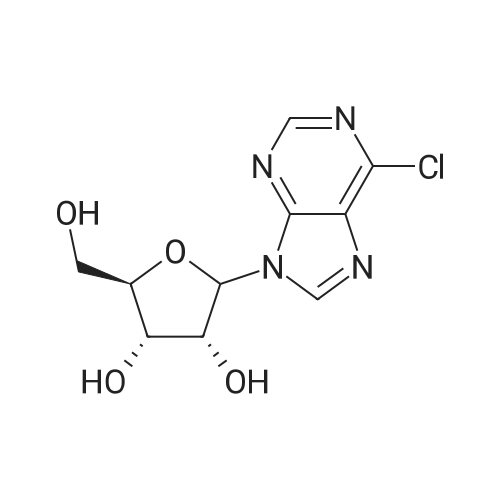
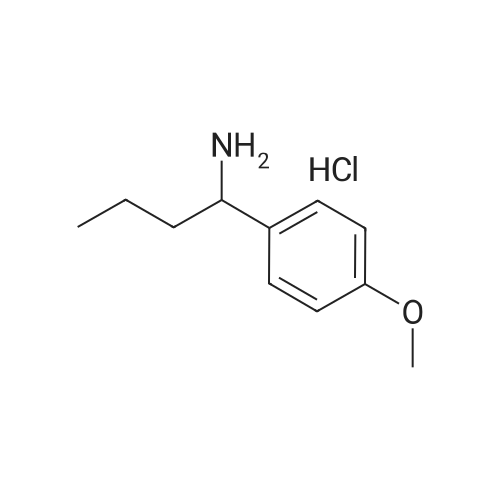
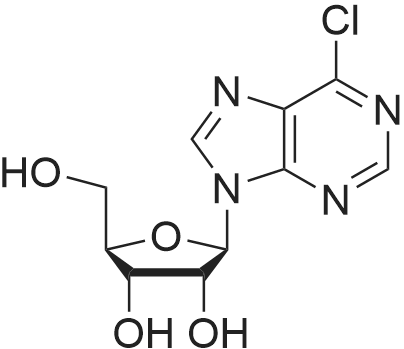
First step, hydroxyamine hydrochloride (619 mg) and NaOAc (1.47 g) were added to a solution of 1-(4-methoxyphenyl)-butanone (800 mg) in EtOH (80 ml). The reaction mixture was stirred at 60°C for 6 h. EtOH was removed under reduced pressure. H2O (40 ml) was added to the residue, and the resulting solution was extracted with EtOAc (3 × 40 ml). The EtOAc of the combined organic layer was removed by rotary evaporation under reduced pressure to yield 1-(4-methoxyphenyl)-butanone oxime (870 mg) as a pale yellowish solid. Second step, a mixture of 1-(4-methoxyphenyl)-butanone oxime (870 mg) and concentrated HCl (2.38 ml) in EtOH (50 ml) was subjected to hydrogenation at atmospheric pressure in the presence of 10% Pd/C (90 mg). The reaction solution was filtered and the filtrate was concentrated. The residue was suspended in EtOAc, and the suspension was filtered to yield 1-(4-methoxyphenyl)-butylamine hydrochloride (965 mg) as a white solid. Third step, a mixture of 1-(4-methoxyphenyl)-butylamine (453 mg, the hydrochloride), 6-chloropurine riboside (200 mg) and triethylamine (3 ml) in PrOH (60ml) was heated to 70°C and reacted for 8 h. After evaporation of the reaction mixture, the residue was separated by column chromatography over silica gel and eluted with CHCl3-CH3OH (20 : 1) to yield N6-[(+/-)-1-(4-methoxyphenyl)-butyl]-adenosine(240 mg) as a white solid: positive ESIMS m/z 430 [M + H]+ and 352 [M + Na]+; 1H NMR (300 MHz, DMSO- d6): the adenosine moiety δ 8.37 (1H, s, H-2), 8.21 (1H, m, -NH), 8.17 (1H, brs, H-8), 5.89 (1H, d, J= 6.3 Hz, H-1'), 5.48 (2H, m, 2×-OH), 5.22 (1H, d, J= 4.8 Hz, -OH), 4.61 (1H, m, H-2'), 4.16 (1H, m, H-3'), 3.98 (1H, m, H-4'), 3.66 (1H, m, H-5'a), 3.56 (1H,, m, H-5'b); the (+/-)-1-(4-methoxyphenyl)-butyl moiety δ 7.36 (2H, d, J = 8.4 Hz, C-2", C-6"), 6.83 (2H, d, J = 8.4 Hz, C-3", C-5"), 5.32 (1H, m, H-7"), 3.76 (3H, s, -OH3), 1.91 (1H, m, H-8"a), 1.72 (1H, m, H-8"b), 1.24 (2H, m, H-9"), 0.86 (3H, t, J = 7.5 Hz, H-10"); 13C NMR (75 MHz, DMSO-d6): the adenosine moiety δ 154.3 (s, C-6), 152.4 (d, C-2), 148.5 (s, C-4), 140.0 (d, C-8), 119.8 (s, C-5), 88.2 (d, C-1'), 86.1 (d, C-4'), 73.6 (d, C-2'), 70.8 (d, C-3'), 61.8 (t, C-5'); the (+/-)-1-(4-methoxyphenyl)-butyl moiety δ 158.1 (s, C-4"), 136.3 (s, C-1"), 127.9 (d, C-2", C-6"), 113.7 (d, C-3", C-5"), 55.1 (q, -OCH3), 52.6 (d, C-7"), 38.2 (t, C-8"), 19.6 (t, C-9"), 13.7 (q, C-10"). |
| 240 mg |
With triethylamine In propan-1-ol at 70℃; for 8h; |
88 Preparation of N6-[(+/-)-1-(4-methoxyphenyl)-butyl]-adenosine
Third step, a mixture of 1-(4-methoxyphenyl)-butylamine (453 mg, the hydrochloride), 6-chloropurine riboside (200 mg) and triethylamine (3 ml) in PrOH (60 ml) was heated to 70° C. and reacted for 8 h. After evaporation of the reaction mixture, the residue was separated by column chromatography over silica gel and eluted with CHCl3-CH3OH (20:1) to yield N6-[(+/-)-1-(4-methoxyphenyl)-butyl]-adenosine (240 mg) as a white solid: positive ESIMS m/z 430 [M+H]+ and 352 [M+Na]+; 1H NMR (300 MHz, DMSO-d6): the adenosine moiety δ 8.37 (1H, s, H-2), 8.21 (1H, m, -NH), 8.17 (1H, brs, H-8), 5.89 (1H, d, J=6.3 Hz, H-1'), 5.48 (2H, m, 2*-OH), 5.22 (1H, d, J=4.8 Hz, -OH), 4.61 (1H, m, H-2'), 4.16 (1H, m, H-3'), 3.98 (1H, m, H-4'), 3.66 (1H, m, H-5'a), 3.56 (1H, m, H-5'b); the (+-)-1-(4-methoxyphenyl)-butyl moiety δ 7.36 (2H, d, J=8.4 Hz, C-2", C-6"), 6.83 (2H, d, J=8.4 Hz, C-3", C-5"), 5.32 (1H, m, H-7"), 3.76 (3H, s, -OH3), 1.91 (1H, m, H-8"a), 1.72 (1H, m, H-8"b), 1.24 (2H, m, H-9"), 0.86 (3H, t, J=7.5 Hz, H-10"); 13C NMR (75 MHz, DMSO-d6): the adenosine moiety δ 154.3 (s, C-6), 152.4 (d, C-2), 148.5 (s, C-4), 140.0 (d, C-8), 119.8 (s, C-5), 88.2 (d, C-1'), 86.1 (d, C-4'), 73.6 (d, C-2'), 70.8 (d, C-3'), 61.8 (t, C-5'); the (+-)-1-(4-methoxyphenyl)-butyl moiety δ 158.1 (s, C-4"), 136.3 (s, C-1"), 127.9 (d, C-2", C-6"), 113.7 (d, C-3", C-5"), 55.1 (q, -OCH3), 52.6 (d, C-7"), 38.2 (t, C-8"), 19.6 (t, C-9"), 13.7 (q, C-10"). |
| 240 mg |
With triethylamine In propan-1-ol at 70℃; for 8h; |
88.3 Third step
1-(4-methoxyphenyl)-butylamine hydrochloride (453 mg) was dissolved in n-propyl alcohol (60 mL), 6-chloropurine nucleoside (200 mg)And triethylamine (3 ml) were added and heated to 70 ° C. to react for 8 h. The solvent was recovered in the reaction solution, chromatographed through a silica gel column, and eluted with chloroform-methanol (20: 1) to give white solid N6-[1-(4-methoxyphenyl)-butyl]-adenosine (240 mg) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping