|
In DMF (N,N-dimethyl-formamide); at 140℃; for 2h; |
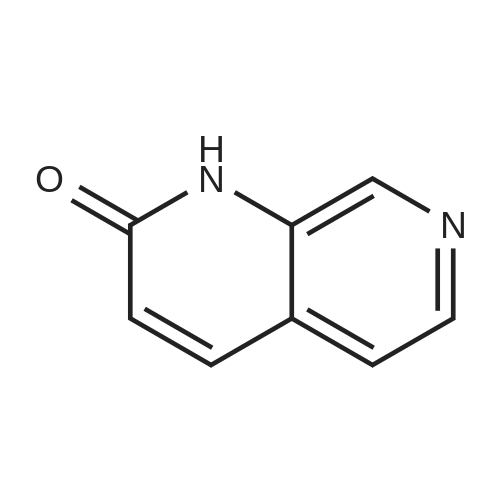
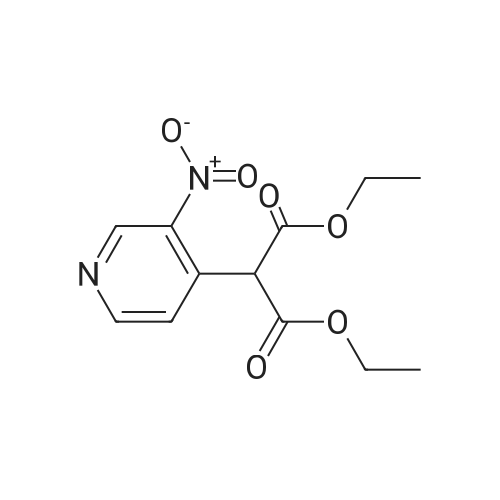
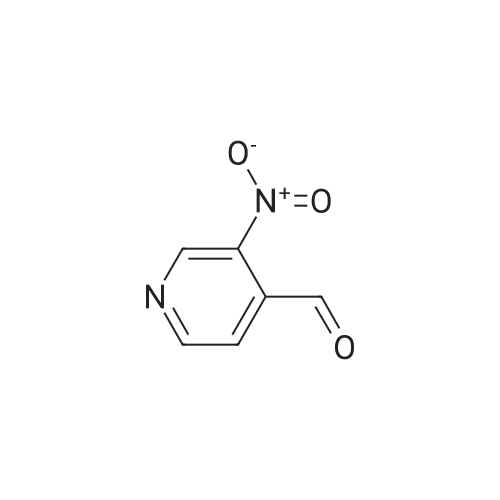
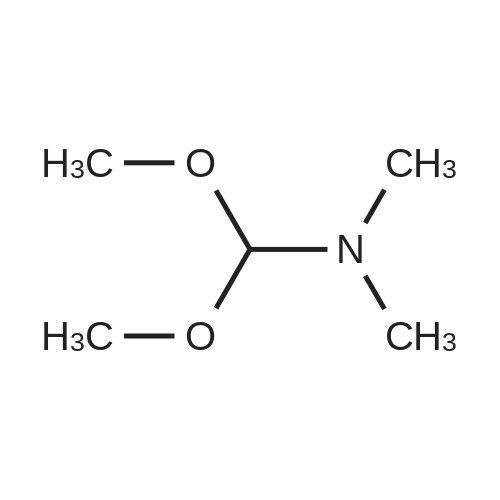
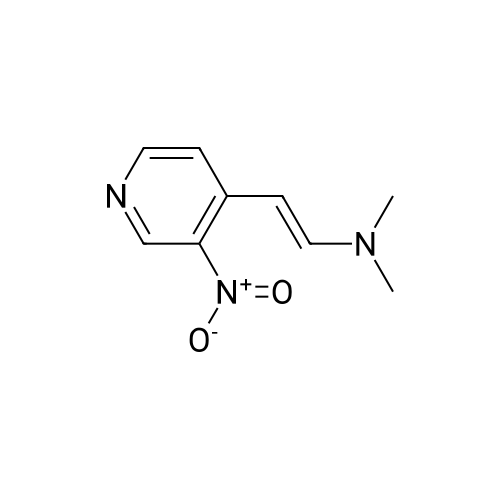
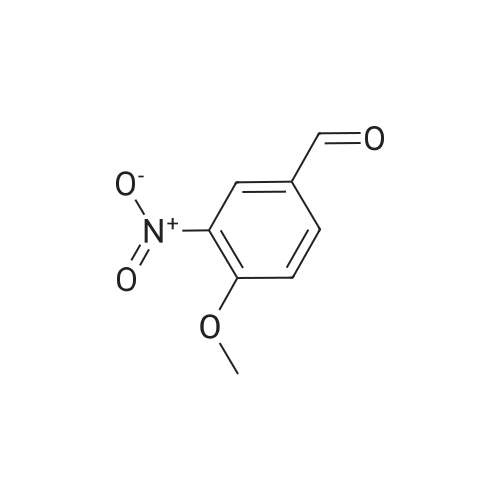
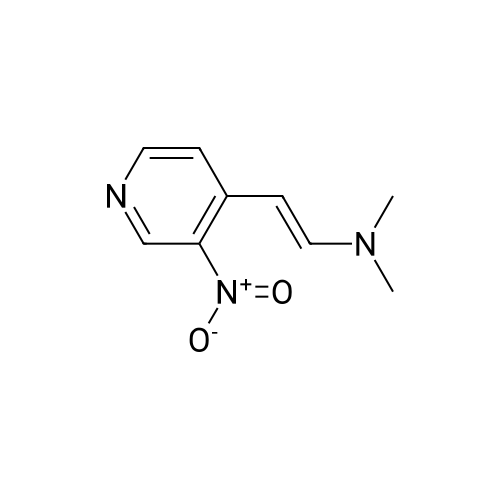
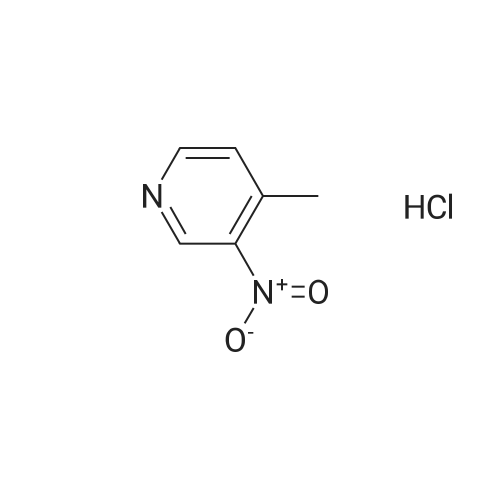
Intermediate 12: (3R/S)-3-AMINO-L-FF (4R)-2, 2-DIMETHVL-1, 3-DIOXOLAN-4-VLLMETHVL -3, 4- dihvdro-1, 7-NAPHTHVRIDIN-2 (1H)-one 4-Methyl-nitropyridine (1.43 g, 10. 36MMOL) was dissolved in dry DMF (5ML) and dimethylformamide dimethyl acetal (2.0 g, 16.8 mmol) was added. The mixture was heated under nitrogen at 140C for 2 hours and then evaporated under reduced pressure to give (E)- N, N-DIMETHYL-2- (3-NITROPYRIDIN-4-YL) ethyleneamine as a dark red solid. This was added in one portion at ambient temperature to a stirred solution of sodium periodate (6.61 g, 31 mmol) in THF/Water 1: 1 (100 mL). After stirring for 2hr at ambient temperature the reaction mixture was filtered and the solid washed with ethyl acetate (100 mL). The washings were combined with the filtrate and organic layer separated. The aqueous was extracted with ethyl acetate x 100 mL) and the combined organic layers were washed with saturated aqueous sodium bicarbonate (100 mL) and brine (100 mL), dried (MGS04) and evaporated under reduced pressure to give a brown solid which was purified by column chromatography (DCM) to give 3-nitroisonicotinaldehyde (960mg, 61%). H NMR 8 : 7.8 (d, 1H); 9.15 (d, 1H); 9.4 (s, 1H) ; 10.4 (s, 1H) Methyl [(TERT-BUTOXYCARBONYL) AMINO] (dimethoxyphosphoryl) acetate (1.73g, 5.82 mmol) was dissolved in dry THF (20 mL) and cooled to-78 C under nitrogen. Tetramethylguanidine (638 mg, 5.55 mmol) was added and the solution stirred at-78 C for a further 10 mins. A solution of 3-nitroisonicotinaldehyde (804 mg, 5.29 mmol) in dry THF (5ML) was added dropwise. The resulting deep red solution was stirred for 2hrs AT-78C, then poured into a mixture of ethyl acetate (100 mL) and water (50 mL). The organic layer was separated, washed with water (2 x 50 mL) and brine (25 mL), dried (MGS04) and evaporated under reduced pressure to give a yellow oil, which was purified by column chromatography (EtOAc: isohexane 1: 1) to give METHYL-2- [ (TERT-BUTOXYCARBONYL) AMINO]-3- (3-NITROPYRIDIN-4- yl) acrylate as a 10: 1 mixture of Z/E isomers (1.57g, 92%). H NMR 8 : 1.3 (s, 9H); 1.4 (s, 0.9H) ; 3.55 (s, 0.3H) ; 3.8 (s, 3H); 6.6 (s, 0. 1H) ; 7.2 (s, 1H) ; 7.25 (d, 0. 1H) ; 7.5 (d, 1H) ; 8.75 (d, 0. 1H) ; 8.8 (s, 1. 1H) ; 8.85 (d, 1H) ; 9.2 (s, 0. 1H) ; 9.25 (s, 1H); MS m/z 322. Methyl 2-[(TERT-BUTOXYCARBONYL) AMINO]-3-(3NITROPYRIDIN-4-YL) ACRYLATE (10: 1 mixture of Z/E isomers) (1.57 g, 4.83 mmol) was dissolved in ethanol and 10% palladium on carbon catalyst (250 mg) was added. The mixture was stirred under 1 atmosphere of hydrogen at ambient temperature for 6 hours. After removing the catalyst by filtration through Celite, the filtrate was concentrated under reduced pressure to give a yellow oil which was purified by column chromatography (Eluent DCM/MEOH gradient 0-10%) to give tert-butyl (2-oxo- 1, 2,3, 4-TETRAHYDRO-1, 7-naphthyridine-3-yl) carbamate (284mg, 22%). H NMR 8 : 1.4 (s, 9H); 3.0 (m, 2H); 4.2 (m, 1H); 7.0 (d, 1H) ; 7.2 (d, LH) ; 8.1 (m, 2H); 10.36 (s, 1H); MS m/z 264. tert-Butyl (2-oxo-1, 2,3, 4-TETRAHYDRO-1, 7-naphthyridine-3-yl) carbamate (284mg) was dissolved in DCM (10 mL) and treated with trifluoroacetic acid (5 mL). After stirring at ambient temperature for 1 hour the reaction mixture was evaporated under reduced pressure and the residue triturated with ether (20 mL) to give a light brown solid which was collected by filtration, washed with ether and dried to give 3-amino-3, 4-DIHYDRO-1, 7-naphthyridin-2 (1H)- one (346 mg, 82%) as a bis trifluroacetate salt. H NMR 8 : 3.2 (m, 2H); 4.3 (m, 1H), 7.4 (d, 1H) ; 8.2 (s, 1H) ; 8.25 (d, 1H) ; 8.6 (b, 3H); 11.0 (s, 1H) Sodium hydride (1.18 g, 60% dispersion in oil, 29.4 mmol) was added to a suspension of (3R/S)-3-Amino-3, 4-DIHYDRO-1, 7-naphthyridin-2 (lH)-one (2.24 g, 9.4 mmol) in dry DMF (40 mL) and the mixture heated to 80 C and stirred for 30 mins. [ (4S)-2, 2-Dimethyl-1, 3- dioxolan-4-yl] methyl methanesulfonate (2 mL, 10.4 mmol) was added and the reaction stirred at 80 C for 19 h. The mixure was cooled, diluted with DCM (450 mL), purified using flash column chromatography (SI02, eluent: DCM to DCM: MeOH, 85: 15 to DCM: MeOH: NH40H, 80: 20: 0.7) and the volatiles removed under reduced pressure to afford the title compound (1. 1 g, 42%) as an oil. LH NMR O : 1.23 (m, 3H), 1.26 (s, 1. 8H), 1. 28 (s, 1.2H), 3.52 (m, 1H), 3.68 (m, 1H), 4.02 (m, 2H), 4.15 (m, 1H), 4.31 (m, 1H), 7.27 (br d, 1H), 8. 21 (d, 1H), 8. 55 (d, 1H) ; MS M/Z 278. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping