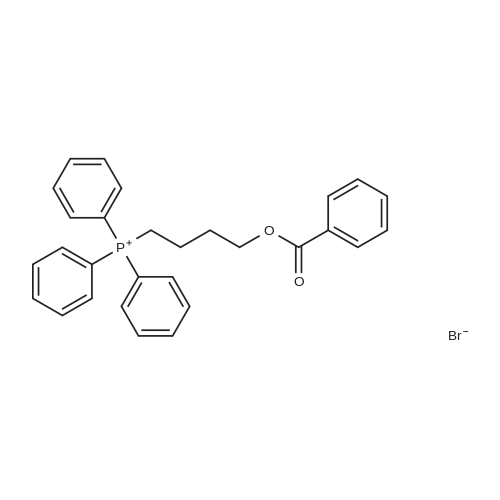
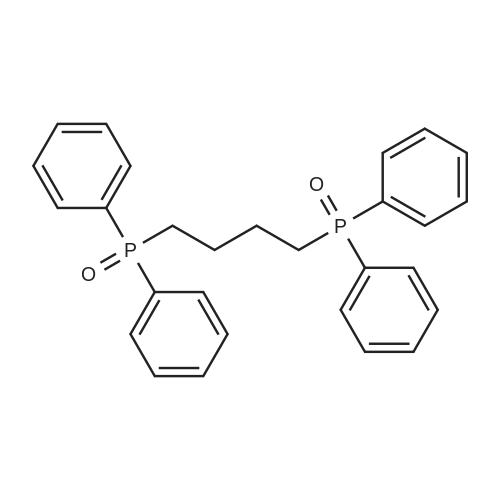
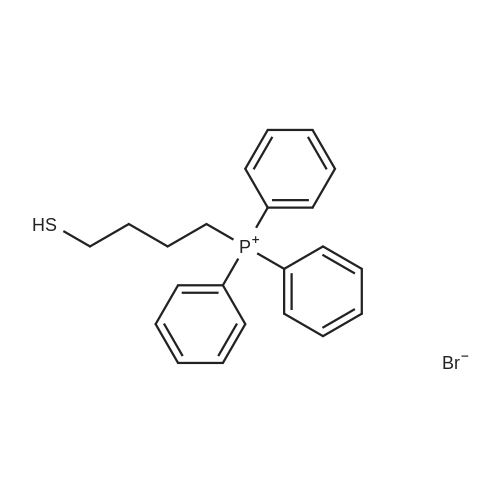
| 73% |
In toluene;Reflux; |
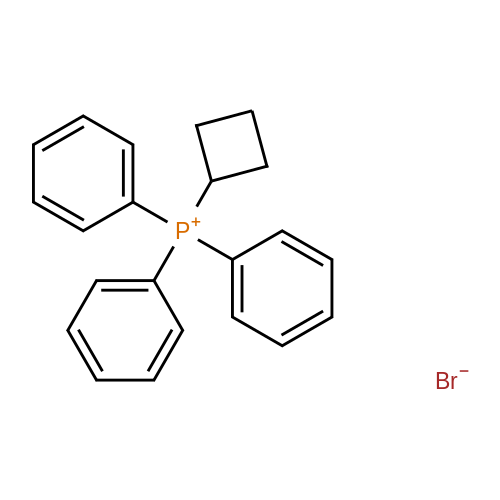
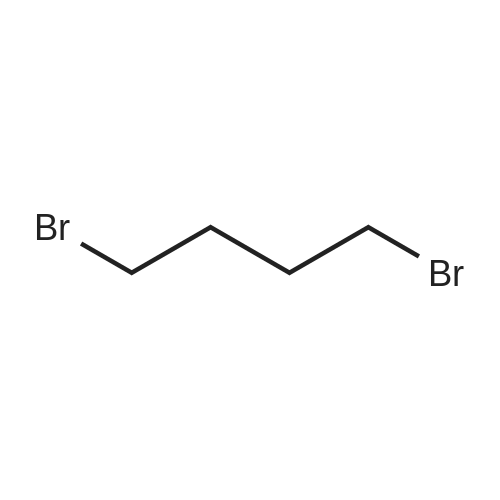
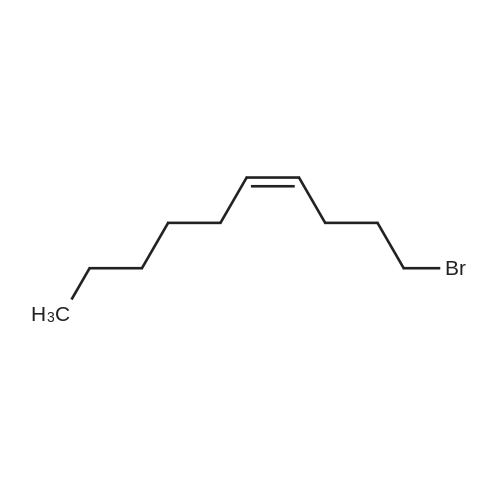
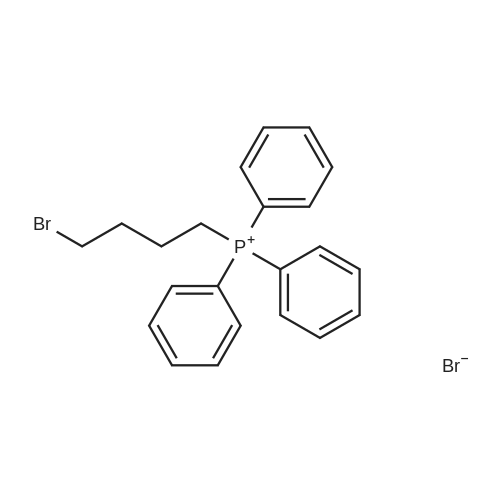
The intermediate compound (4-Bromobutyl)triphenylphosphonium bromide (compound (12)) depicted in Reaction 3 above was prepared by placing lOg (46.3 mmol) of 1,4-dibromobutane (compound (11)) and 12.lg PPh3 (46.3 mmol) in dry toluene (74mL), and the mixture heated to reflux and boiled overnight. The solid that formed was filtered, washed with toluene and dried under vacuum to provide the product compound (12) as a white solid. Yield: 16.lg (73%). ?HNMR (300 MHz, CDC13): oe 7.84-7.68 (m, 15H), 4.03-3.93 (m, 2H), 3.58 (t, J= 6 Hz, 2H), 2.36-2.28 (m, 2H), 1.89-1.76 (m, 2H). APCI[M+H] 397 (M-Br), 399 (M+2-Br). |
| 40% |
In benzene; at 80℃; for 20h; |
The synthesis was similar to that described in [32] . Namely, a solution of 1,4-dibrombutyl (1.1 ml, 12 mmol) and triphenylphosphine (2.62 g, 10 mmol) in benzene (0.5 ml) was heated at 80 C during 20 h in a tightly closed flask. Then the reaction mass was cooled to 20 C, diluted with dichloromethane and evaporated to dryness. The residue was dissolved in a minimal volume of dichloromethane, then an excess of diethyl ester was added and the suspension formed was kept at 4 C until the solution became clear. Then the liquid phase was decanted, the residue was dissolved again in dichloromethane and treated with diethyl ether to complete precipitation. This procedure was repeated three times. Finally the residue was dissolved in a minimal volume of the solvent system ethanol-dichloromethane (1:5) and applied to a chromatographic silica gel column (MN Kieselgel 60, 240-400 mesh) in the same solvent system as an eluent. Detection was carried out using TLC by UV-absorbance and Dragendorf reaction. Fractions with the same chromatographic mobilities were combined and evaporated in vacuo (yield 1,8 g, 40%). ESI-MS: calculated for C22H23PBr 398.3, observed 399.2 (M + H)+. |
|
In toluene; for 24h;Reflux; Inert atmosphere; |
The procedure for grafting the polymer withasymmetrical dication IL is shown in Scheme 1. First, (4-bromobutyl)triphenylphosphonium bromide) ([Ph3PC4H8Br]Br)was synthesized according to the following procedure (Scheme 1a):A mixture of triphenylphosphine (10 mmol), 1,4-dibromobutane (10 mmol), and toluene (20 mL) was under reflux in a round bottom flask (100 mL) for 24 h in a nitrogen atmosphere. The as-resulted mixture was cooled down and subject to filtration. The obtained residue was washed three times with diethyl ether, followed by drying at 60 C for 12 h in vacuum to give [Ph3PC4H8Br]Br as a white solid. 1H NMR (400 MHz, DMSO-d6): delta = 7.90-7.73 (m, 15 H), 3.63-3.32 (m, 4 H), 2.49-2.47 (m, 2 H), 1.99-1.64 (m, 2 H). 13C NMR (100.6 MHz, DMSO-d6): delta = 135.4, 130.8, 119.3, 40.2, 34.2, 33.2, 20.8, 19.6. IR (neat): n = 3074, 3057, 2931, 2885, 2862, 1585, 1485, 1436, 750, 690 cm-1. HR-MS (QTOF): m/z = 399.0864, calcd. for C22H25PBr (M + H): 399.0877. We also synthesized butyltriphenylphosphonium bromide ([Ph3P-C4H9]Br) using the same method. |
|
In toluene; at 110℃; for 24h;Inert atmosphere; |
The preparation procedure of CS-grafted 1-butyl-triphenyl phosphonium bromide (denoted hereinafter as CS-[BuPh3P]Br) is illustrated in Scheme 1 . First, a solution of triphenylphosphine (10 mmol) in 10 mL toluene was dropped slowly into 10 mL toluene solution containing 1,4-dibromobutane (10 mmol). The mixture was stirred at 110 C for 24 h under a nitrogen atmosphere. After the reaction, the mixture was cooled down to room temperature (RT), and the resultant crude solid was filtered out and washed three times with diethyl ether, then dried under vacuum at 60 C for 12 h to give 4-bromobutyl-triphenyl phosphonium bromide ([BrBuPh3P]Br) as a white solid. |
|
In benzene; at 80℃; |
Dibromoalkanes (60, 61) were heated with (triphenyl)phosphine in benzene at 80C, and the obtained bromoalkyl(triphenyl)phosphonium bromides (62, 63) were reacted with fluorescein in the presence of sodium carbonate. As a result, bromides of {4-[2-(6-hydroxy-3-oxo-3H- xanthen-9-yl)benzoyloxy]butyl} (triphenyl)phosphonium (64) and {10-[2-(6-hydroxy-3-oxo-3H- xanthen-9-yl)benzoyloxy]decyl}(triphenyl)phosphonium (65). These compounds are fluorescent uncouplers possessing neuroprotective and nephroprotective properties. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping