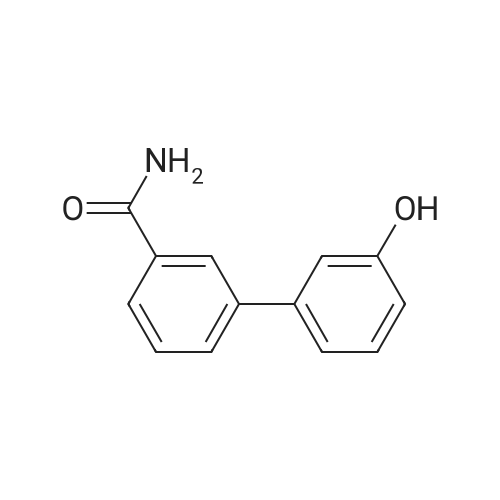
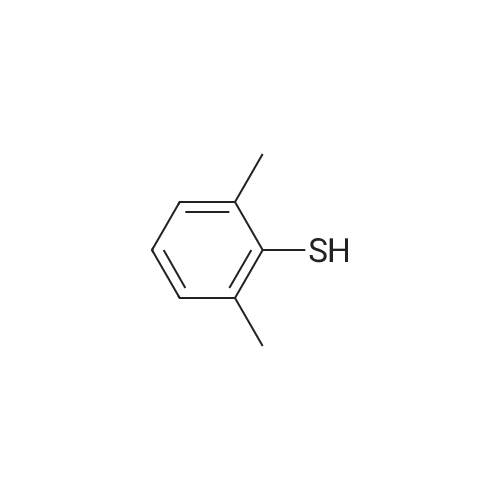
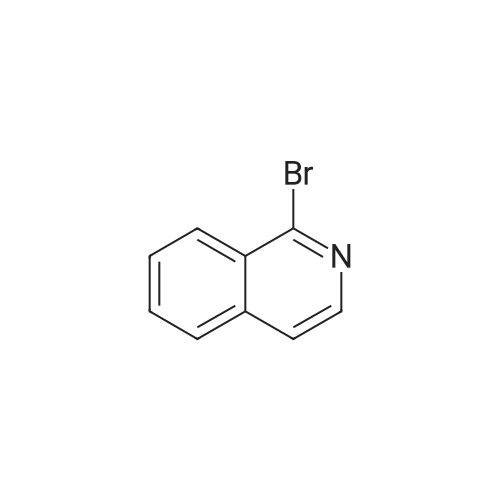
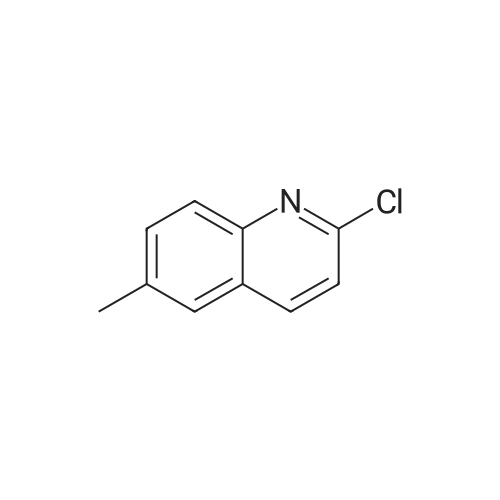
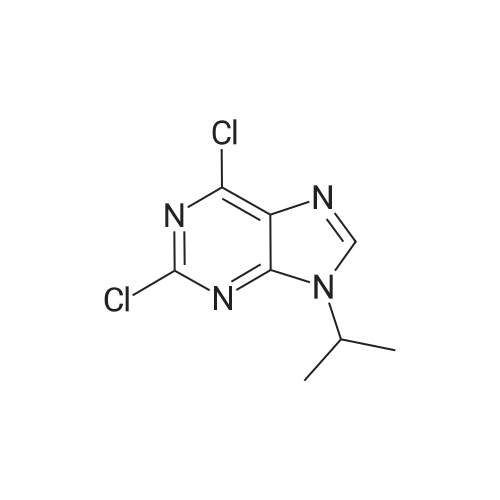
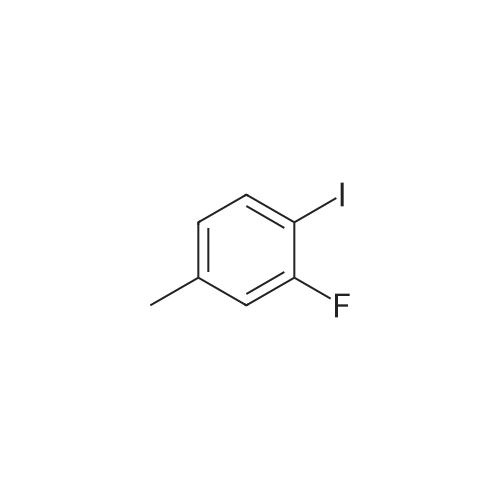
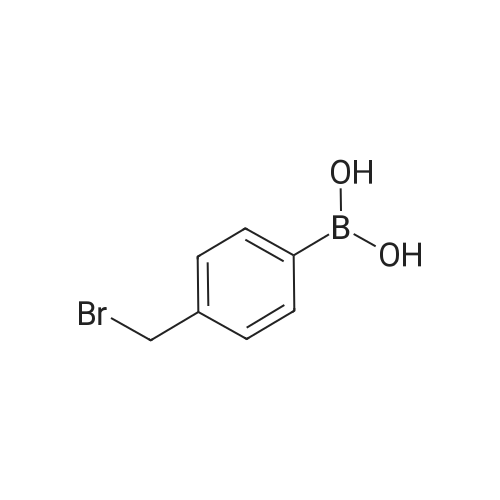
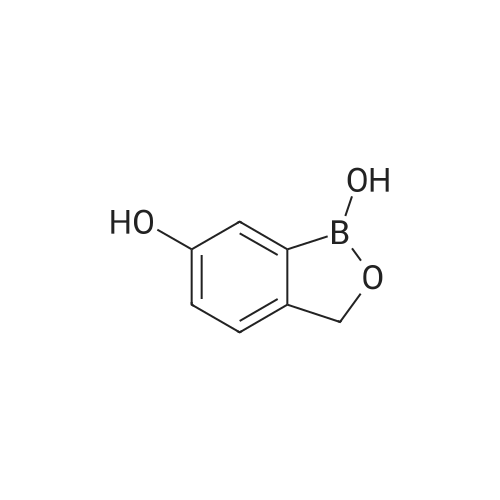
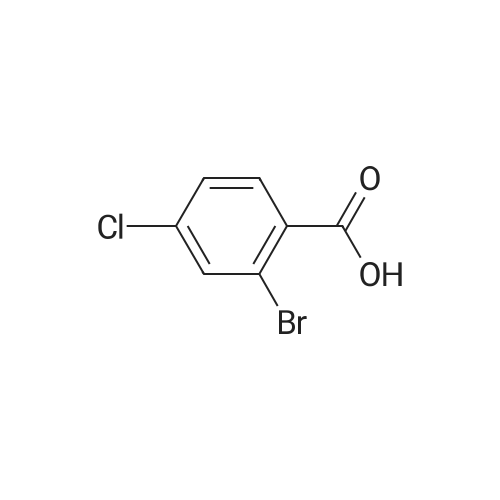
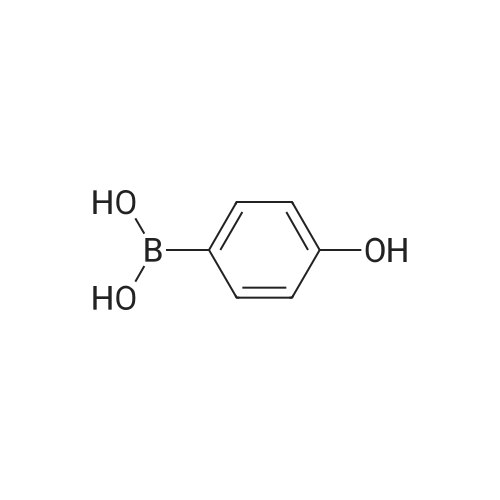
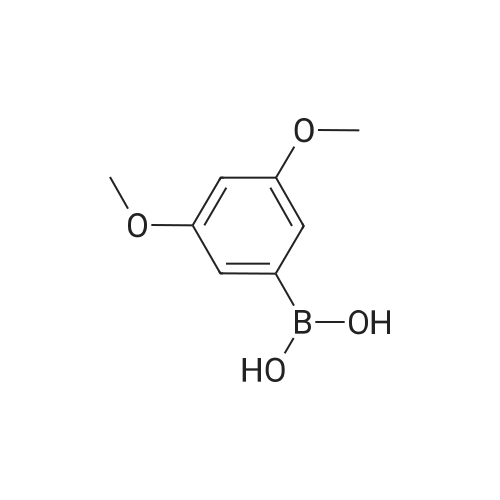
| 100% |
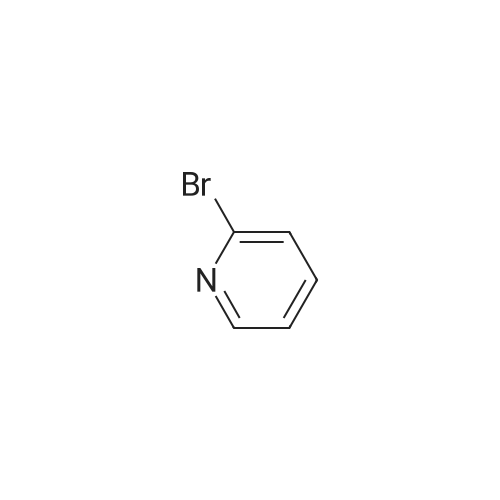
With sodium carbonate In ethanol; water; toluene at 90℃; for 5h; |
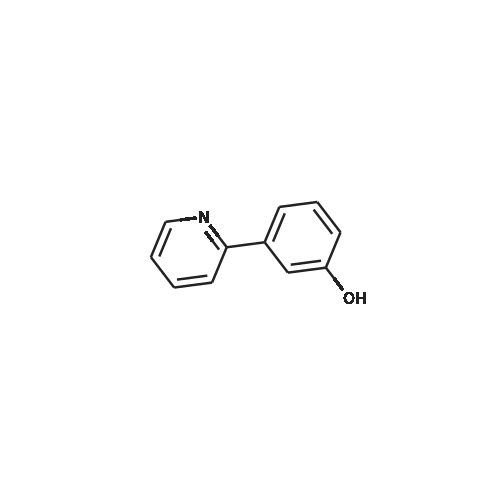
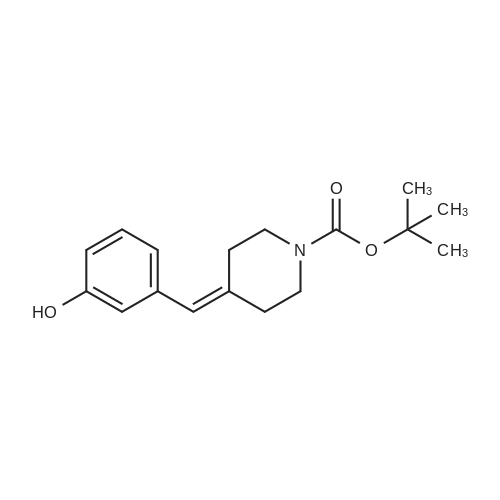
7 INTERMEDIATE 7: tert-butyl 4- [ {4- [ (DIETHYLAMINO) carbonyl] phenyl} (3- hydroxyphenyl) methylene] piperidine-1-carboxylate
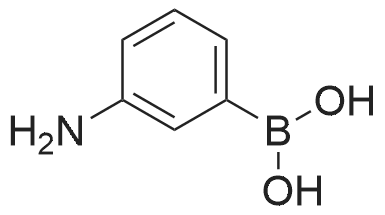
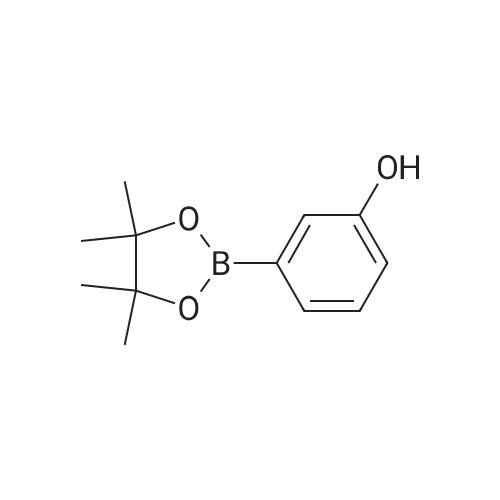
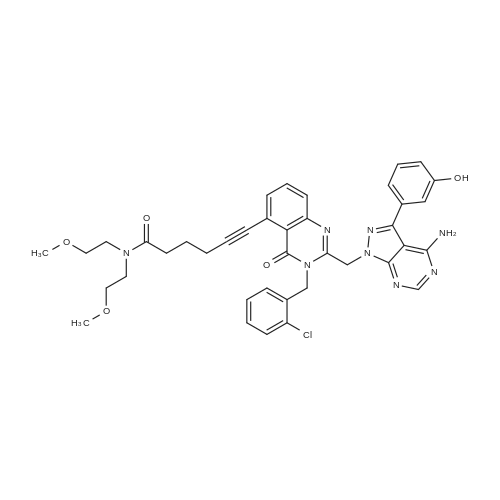
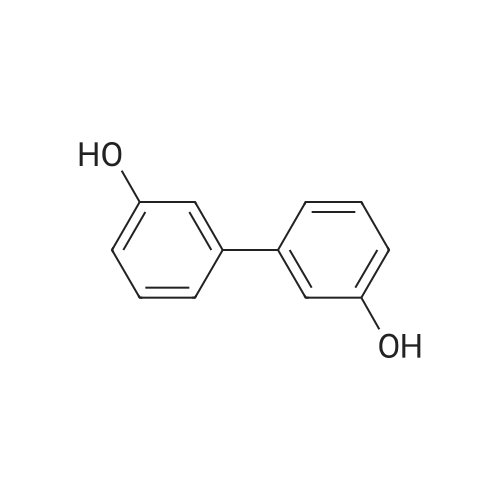
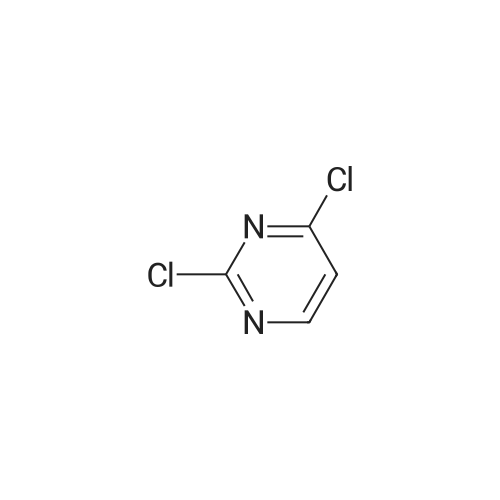
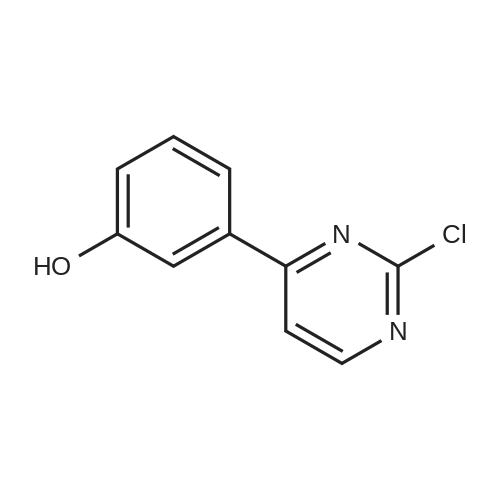
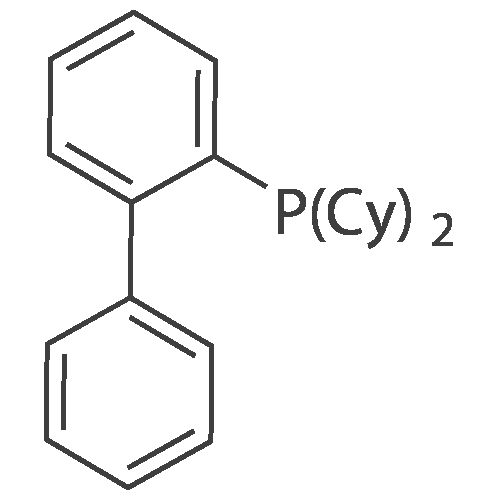
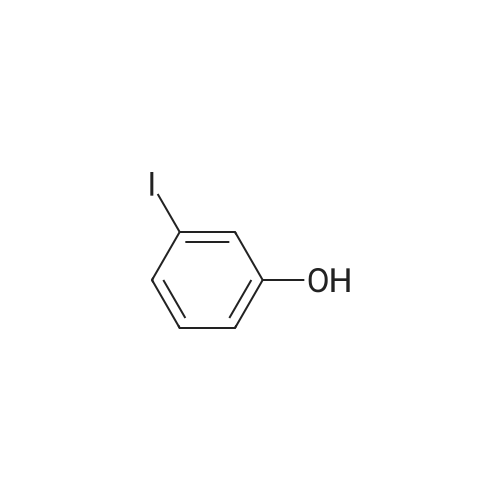
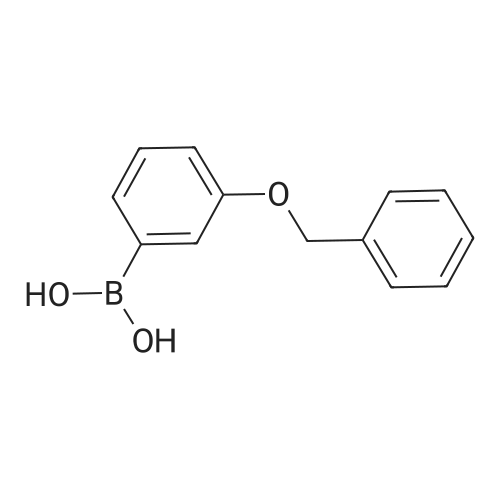
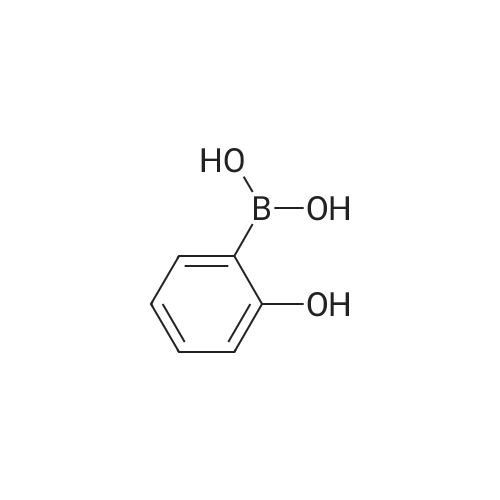
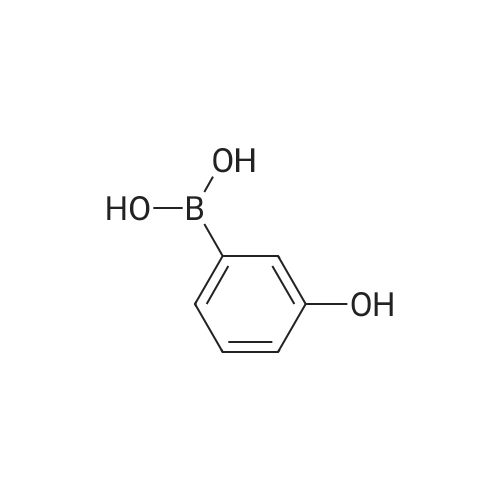
A solution of INTERMEDIATE 5 (4.08 g, 9.04 mmol), 3-hydroxyphenylboronic acid (1.97 g, 14.3 mmol) and aqueous 2N sodium carbonate (11.3 mL, 22.6 mmol) in a 1: 1 mixture of toluene/ethanol (200 mL) was degassed for 20 minutes. Palladium tetrakistriphenylphosphine (1.05 g, 0.909 mmol) was added and the reaction mixture was purged with nitrogen and heated to 90 °C. After 5 h, the reaction was cooled to room temperature and saturated aqueous ammonium chloride was added. The mixture was extracted with two portions of ethyl acetate and the combined organic extracts were dried (NA2SO4), filtered and concentrated. The residue was purified by flash chromatography eluting with 0% to 100% ethyl acetate in hexanes to yield INTERMEDIATE 7 as a white solid (4.24 g, 100%). 1H NMR (400MHZ, CDC13) 8 1.10 (t, J = 7.42 Hz, 3H), 1.20 (t, J = 7.03 Hz, 3H), 1.42 (s, 9H), 2.25-2. 33 (m, 4H), 3.23-3. 31 (m, 2H), 3.39-3. 46 (m, 4H), 3.46-3. 54 (m, 2H), 6.51 (dd, J = 2.15, 1.56 Hz, 1H), 6.57 (ddd, J = 7.62, 1.56, 0.98 Hz, 1H), 6.62 (ddd, J = 8.20, 2.54, 0.98 Hz, 1H), 7.06-7. 12 (m, 1H), 7.19 (d, J = 8.40 Hz, 2H), 7.29 (d, J = 8.40 Hz, 2H). |
| 100% |
With sodium carbonate In ethanol; water; toluene at 90℃; for 5h; |
6 INTERMEDIATE 6: TERT-BUTYL 4- [ {4- [ (DIETHYLAMINO) CARBONYL] PHENYL} (3- hydroxyphenyl) methylene] PIPERIDINE-1-CARBOXYLATE
To a flask containing INTERMEDIATE 5 (4.08 g, 9.04 mmol) was added toluene (100 ML), ethanol (100 mL), 3-hydroxyphenylboronic acid (1. 97 g, 14.3 MMOL), and aqueous 2N sodium carbonate (11. 3 mL, 22.6 MMOL). The solution was degassed for 20 minutes, then palladium tetrakistriphenylphosphine (1. 05 g, 0.909 mmol) was added. The reaction mixture was purged with nitrogen and heated to 90 °C. After 5 h, the reaction was cooled to rt and saturated aqueous ammonium chloride was added. The mixture was extracted with two portions of ethyl acetate and the combined organic extracts were dried (NA2SO4), filtered and concentrated. The residue was purified by flash chromatography, eluting 0% to 100% ethyl acetate in hexanes, to yield INTERMEDIATE 6 as a colourless solid (4.24 g, 100%).'H NMR (400MHZ, CDCL3) 6 1. 10 (t, J = 7.42 Hz, 3H), 1.20 (t, J = 7.03 Hz, 3H), 1.42 (s, 9H), 2.25-2. 33 (M, 4H), 3.23-3. 31 (M, 2H), 3.39-3. 46 (M, 4H), 3.46-3. 54 (M, 2H), 6.51 (dd, J = 2.15, 1. 56 Hz, 1H), 6.57 (ddd, J = 7.62, 1.56, 0.98 Hz, 1H), 6.62 (ddd, J = 8. 20, 2.54, 0.98 Hz, 1H), 7.06-7. 12 (M, 1H), 7.19 (d, J = 8.40 Hz, 2H), 7.29 (d, J = 8. 40 Hz, 2H). |
| 100% |
With sodium carbonate In ethanol; water; toluene at 20 - 90℃; for 5.33333h; |
INTERMEDIATE 7: tert-butyl 4-[{4-[(DIETHYLAMINO) CARBONYL] PHENYL} (3- hydroxyphenyl) methylene] piperidine-I-carboxylate
To a flask containing INTERMEDIATE 5 (4.08 g, 9.04 mmol) was added toluene (100 mL), ethanol (100 mL), 3-hydroxyphenylboronic acid (1.97 g, 14.3 MMOL), and aqueous 2N sodium carbonate (11. 3 mL, 22.6 MMOL). The solution was degassed for 20 minutes, then palladium tetrakistriphenylphosphine (1. 05 g, 0.909 mmol) was added. The reaction mixture was purged with nitrogen and heated to 90 °C. After 5 h, the reaction was cooled to rt and saturated aqueous ammonium chloride was added. The mixture was extracted with two portions of ethyl acetate and the combined organic extracts were dried (NA2SO4), filtered and concentrated. The residue was purified by flash chromatography, eluting 0% to 100% ethyl acetate in hexanes, to yield INTERMEDIATE 7 as a colourless solid (4.24 g, 100%). 1H NMR (400MHZ, CDCL3) No. 1.10 (t, J = 7.42 Hz, 3H), 1.20 (t, J = 7.03 Hz, 3H), 1.42 (s, 9H), 2.25-2. 33 (m, 4H), 3.23-3. 31 (m, 2H), 3.39-3. 46 (m, 4H), 3.46-3. 54 (m, 2H), 6.51 (dd, J = 2.15, 1.56 Hz, 1H), 6.57 (ddd, J = 7.62, 1.56, 0.98 Hz, 1H), 6.62 (ddd, J = 8. 20, 2.54, 0.98 Hz, 1H), 7.06-7. 12 (m, 1H), 7.19 (d, J = 8.40 Hz, 2H), 7.29 (d, J = 8. 40 Hz, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping