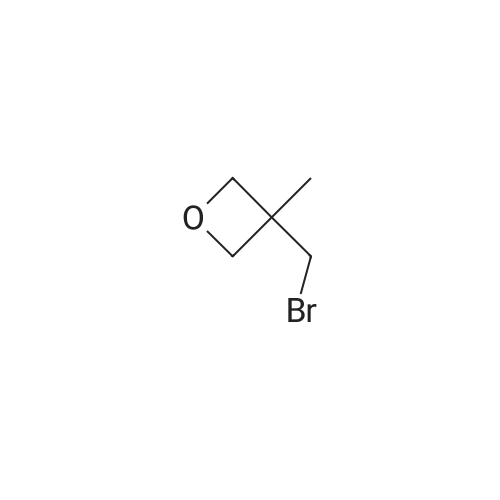
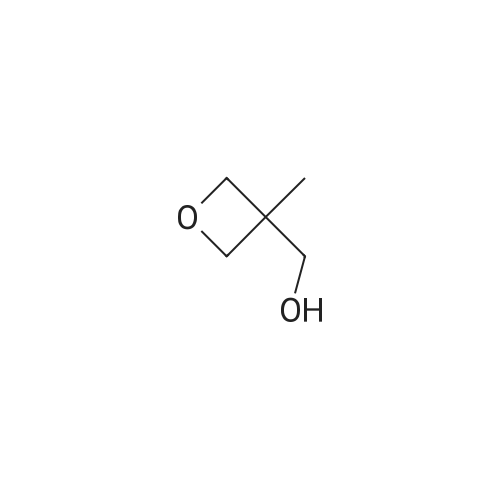
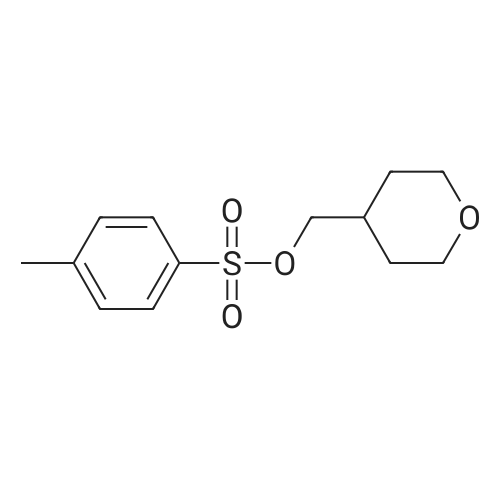
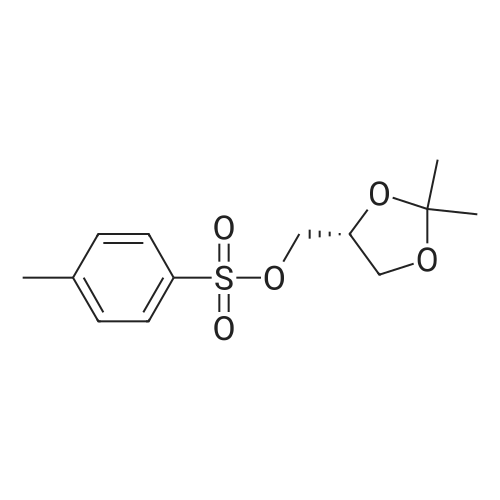
| 74.7% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 5h; |
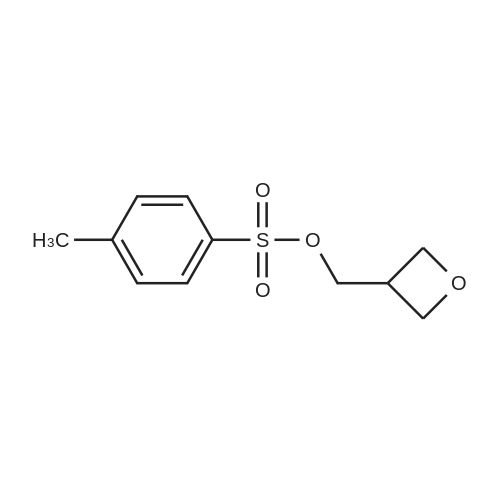
Step 1 a Synthesis of 3-Methyloxetan-3-yl)methyl 4-methylbenzenesulfonate To a solution of (3-methyloxetan-3-yl)methanol (1 g, 9.79 mM) in DCM (15 ml), triethylamine (2.71 ml, 19.58 mM) was added at 0 QC followed by the addition of 4- methylbenzene-1 -sulfonyl chloride (1 .867 g, 9.79 mM). The reaction mixture was stirred at RT for 3h to 5h. The reaction mixture was then quenched with water, extracted with ethyl acetate and purified by column chromatography to afford the title compound (3-methyloxetan-3-yl)methyl 4-methylbenzenesulfonate (1 .875 g) as a white solid. Yield: 74.7%; 1 H NMR (DMSO-d6, 300 MHz): delta 7.82 (d, J=8.1 Hz, 2H), 7.51 (d, J=8.1 Hz, 2H), 4.25 (d, J=5.7 Hz, 2H), 4.19 (d, J=6.0 Hz, 2H), 4.1 1 (s, 2H), 2.43 (s, 3H), 1 .18 (s, 3H); MS (ESI): m/z 279.0 (M+Na). |
| 72% |
With pyridine; at 0 - 20℃; for 1.75h;Inert atmosphere; |
Exam le 429: A solution of p-toluenesulfonyl chloride (74.36 g, 0.39 mol, 1 .5 equiv) in anhydrous pyridine (300 mL) is dropwise added 3-methyl-3-oxetane- methanol (26.52 g, 0.26 mol) over 10 min at 0C under argon. After 5 min, the reaction mixture is allowed to warm to room temperature with the stirring is continued for an additional 1 .5h. The mixture is then slowly added to a vigorously stirring mixture of milliQ water 800 mL and crushed ice 800 g for 30 min. Then the white precipitate is collected on whatman filter No.1 and washed with cold water (300 mL). The product is dried under high vacuum to obtain a white power of oxetane tosylate 29 (47.88 g 72% yield). 1H NMR (300 MHz, CDCI3) 51 .28 (s, 3H), 2.43 (s, 3H), 4.08 (s, 2H), 4.29-4.35 (m, 2H), 7.34 (d, J=7.8 Hz, 2H), 7.78 (d, J=7.5 Hz, 2H); 13C NMR (75 MHz, CDCI3) 520.85, 21 .86, 39.45, 74.50, 79.13, 128.15, 130.22, 132.81 , 145.34; LC/MS m/s [M+H]+164.9. |
| 50% |
With pyridine; at 0℃; for 2h; |
Synthesis of Intermediate (3-methyloxetan-3'Vl)methyl 4-methylbenzenesulfonate (1-16a):p-Toluene sulfonyl chloride (5.6 g, 29.41 mmol) was added to a cooled solution of (3-methyloxetan-3-yl)methanol (2 g, 19.60 mmol) in pyridine (25 mL) at 0C and the resulting reaction mass was stirred at 0C for 2 hours. The reaction was monitored by TLC (20% ethyl acetate in hexane). The reaction mass was poured into ice-water, stirred for 30 minutes, the solid formed was collected by filtration, washed with water and dried under reduced pressure to afford 2.5 g of the product (50% yield).1HNMR (CDCI3, 300MHz): delta 7.8 (d, 2H), 7.4 (d, 2H), 4.4 (m, 4H), 4.1 (s, 2H), 2.5 (s, 3H), 1.3 (s, 3H). LCMS: 99.13%, m/z = 256 (M+1) |
| 50% |
With pyridine; In methanol; at 0℃; for 2h; |
p-Toluene sulfonyl chloride (5.6 g, 29.41 mmol) was added to a cooled solution of (3-methyloxetan-3-yl)methanol (2 g, 19.60 mmol) in pyridine (25 mL) at 0 C. and the resulting reaction mass was stirred at 0 C. for 2 hours. The reaction was monitored by TLC (20% ethyl acetate in hexane). The reaction mass was poured into ice-water, stirred for 30 minutes, the solid formed was collected by filtration, washed with water and dried under reduced pressure to afford 2.5 g of the product (50% yield).1HNMR (CDCl3, 300 MHz): delta 7.8 (d, 2H), 7.4 (d, 2H), 4.4 (m, 4H), 4.1 (s, 2H), 2.5 (s, 3H), 1.3 (s, 3H). LCMS: 99.13%, m/z=256 (M+1) |
| 47% |
With pyridine; In dichloromethane; at 0 - 20℃; for 16h; |
To a solution of scheme 9-1 compound 51 (10.2 g, 100 mmol) and pyridine (60 mL) in anhydrous DCM (60 mL) at 0 C was added TsC1 (22.92 g, 120 mmol). The reaction mixture was stirred at room temperature for 16 h and then quenched with water (100 mL). The resulting mixture was extracted with DCM (150 mL x 2). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered, and then concentrated. The residue was purified by column chromatography on silica gel to afford scheme 9-1 compound S2 (12 g, 47% yield). LC-MS: m/z 257 (M+H)t |
| 43% |
With dmap; triethylamine; In dichloromethane; at 20℃; for 3h; |
To a solution of (3-methyloxetan-3-yl)methanol (1.02 g, 10 mmol) inDCM (20 ml) was added N,N-dimethylpyridin-4-amine (122 mg, 1 mmol), TEA (2.0 g, 20mmol) and 4-methylbenzenesulfonyl chloride (1.9 g, 10 mmol). The mixture was stirred at rtfor 3 h, concentrated, and the residue purified by column chromatography eluting with ethylacatate in petroleum ether (1/4) to afford (3-methyloxetan-3-yl)methyl 4-methylbenzenesulfonate (1.1 g, 43% yield). LCMS (ESI) m/z: 257.4 (M + 1t. |
|
With pyridine; at 20℃; for 2h; |
Example 9; Synthesis of iy-(l-cyanocyclopropyl)-2(.S)-[2,2-difluoro-2-pyridin-2-yl-l(5)-(4- fluorophenyl)ethylamino]pentamide EPO <DP n="62"/>Step l(3-Methyloxetan-3-yl)methanol (20 g, 190 mmol) was added to a solution of toluene sulfonylchloride (54.3g, 285 mmol) in dry pyridine (100 ml) and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was poured into crushed ice and stirred vigorously for 30 min. The precipitates were filtered and dried to give toluene-4-sulfonic acid 3-methyl-oxetan- 3-ylmethyl ester (37g) as a white powder. |
|
With triethylamine; In dichloromethane; at 0 - 20℃; |
To a solution of (3-methyl-oxetan-3-yl)-methanol (3 mL, 30 mmol) and NEt3 (4.7 mL, 33 mmol) in CH2Cl2 (30 mL) is added toluene-4-sulfonyl chloride (6.4 g, 33 mmol) at 0 C. The reaction is allowed to warm to RT overnight. For workup, a saturated solution of NaHCO3 is added and the mixture is extracted with EtOAc. The crude product is purified by flash column chromatography (n-hexane/EtOAc 1:1) to give the title compound. |
|
With dmap; In dichloromethane; at 20℃; for 1h; |
EXAMPLE 108a Preparation of intermediate toluene-4-sulfonic acid 3-methyl-oxetan-3-ylmethyl ester To a mixture of (3-methyl-oxetan-3-yl)-methanol (10.2 g, 0.1 mol) and DMAP (18.3 g, 0.15 mol) in DCM (100 mL) was added 4-methyl-benzenesulfonyl chloride (19 g, 0.1 mol). The mixture was stirred at room temperature for 1 h, then filtered. The filtrate was washed with HCl aq. (1 M) and water, dried over anhydrous Na2SO4 and concentrated to give the title compound (18 g). |
|
With pyridine; for 1.5h; |
p-Toluenesulfonyl chloride (57.2 g, 300 mmol) was dissolved in dry pyridine (400 mL) under nitrogen atmosphere. 3-methyl-3-(hydroxymethyl)oxetane (20.4 g, 200 mmol) was added slowly, and the solution was stirred for 1.5 h. Crushed ice (400 g) was then added to vigorously stirring mixture, which was allowed to stir for an additional 0.5 h. The white precipitate was then collected on Whatman filter paper No.1 and washed with cold water. The product was dried under high vacuum to obtain 3-methyl-3-(toluenesulfonyl-oxymethyl)oxetane (oxetane tosylate) as a white powder of oxetane tosylate. |
|
With pyridine; at 20℃; |
To a solution of /j-toluenesulfonyl chloride (14.3 g, 75 mmol) in pyridine (60 niL) is slowly added 3-methyl-3-oxetanemethanol A3a (5.1 g, 50 mmol) over 10 min. The resulting suspension is stirred at rt for 1.5 h and poured into vigorously stirred ice water (300 mL). The slurry is stirred for 45 min. The solid is collected by filtration, washed with ice-cold H2O (100 mL), and dried under high vacuum to afford (3- methyloxetan-3-yl)methyl 4-methylbenzenesulfonate A3b as a white powder. The compound is used in the next step without further purification. 1H-NMR (400 MHz, CDCl3) delta = 7.83 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 4.39 (d, J = 6.4 Hz, 2H), 4.36 (d, J = 6.4 Hz, 2H), 4.13 (s, 2H), 2.49 (s, 3H), 1.33 (s, 3H). |
|
With pyridine; for 4.5h;Inert atmosphere; Cooling with ice; |
Under nitrogen atmosphere, a mixture of 1.5 g of (3- methyloxetan-3-yl ) methanol of the following formulaand 150 ml of pyridine, 33 g of p-toluenesulfonyl chloride was added, and stirred for 4.5 hours under ice-cooling. To the reaction mixture, water was added and extracted with ethyl acetate 2 times. The organic layer was washed with 1 mol/1 of hydrochloric acid and brine successively. The organic layer was dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography to give 28 g of p-toluenesulfonic acid 3- methyloxetan-3-ylmethyl ester of the following formula.XH NMR (CDC13) : delta ppm: 7.81 (2H, d) , 7.37 (2H, d) , 4.36 (4H, dd) , 4.11 (2H, s) , 2.47 (3H, s), 1.31 (3H, s) |
| 1.05 g |
With 1,4-diaza-bicyclo[2.2.2]octane; In dichloromethane; at 0℃; for 0.25h; |
EXAMPLE 17 3-Methyl-3-oxetanylmethyl-4-methylbenzene-sulfonate At 0 C., to a solution of 3-methyl-3-oxetanyl methanol (507 mg, 4.97 mmol, commercially available) in CH2Cl2 (15 mL) was added DABCO (1.12 g, 9.94 mmol) and TsCl (1.09 g, 5.72 mmol) was then added dropwise, the mixture was stirred for 15 minutes. After filtration, the filter cake was washed with CH2Cl2 and the filtrate was washed twice with water. The organic phase was dries over anhydrous sodium sulphate. The solvent was evaporated under vacuum to afford the residue (1.05 g), which was used for the next step without further purification. |
|
With 1,4-diaza-bicyclo[2.2.2]octane; In dichloromethane; at 0℃; for 0.25h; |
Example 17 3-Methyl-3-oxetanylmethyl-4-methylbenzene-sulfonate At 0C, to a solution of 3-methyl-3-oxetanyl methanol (507 mg, 4.97 mmol, commercially available) in CH2Cl2 (15 mL) was added DABCO (1.12 g, 9.94 mmol) and TsCl (1.09 g, 5.72 mmol) was then added dropwise, the mixture was stirred for 15 minutes. After filtration, the filter cake was washed with CH2Cl2 and the filtrate was washed twice with water. The organic phase was dries over anhydrous sodium sulphate. The solvent was evaporated under vacuum to afford the residue (1.05 g), which was used for the next step without further purification. |
|
With pyridine; In dichloromethane; at 20℃; for 4h; |
p-Toluenesulfonyl chloride (2.17 g, 11.4 mmol) is dissolved in CH2CI2 (9.5 mL) at it Pyridine (1.53 mL, 19 mmol) is added, followed by 3-methyl-3- oxetanemethanol (0.977 mL, 9.5 mmol). The sol. is stirred at rt for 4 h. The sol. is diluted with CH2CI2 and washed with aq. 0.1 M HCI and with aq. sat. NaHC03. The org. layer is dried over Na2S04, filtered, and the solvents are removed under reduced pressure. Purification of the crude by automated FC (Combiflash, EtOAc/heptane 0: 100? 60:40) yields the title product. LC-MS: tR = 0.80 min, MH+ = 257.17 (conditions 3). |
|
With pyridine; In dichloromethane; at 20℃; for 4h; |
(3-Methyloxetan-3-yl)methyl 4-methylbenzenesulfonate. p-Toluenesulfonyl chloride (2.17 g, 11.4 mmol) is dissolved in CH2CI2 (9.5 mL) at rt. Pyridine (1.53 mL, 19 mmol) is added, followed by 3-methyl-3- oxetanemethanol (0.977 mL, 9.5 mmol). The sol. is stirred at rt for 4 h. The sol. is diluted with CH2CI2 and washed with aq. 0.1 M HCI and with aq. sat. NaHCC>3. The org. layer is dried over Na2SC>4, filtered, and the solvents are removed under reduced pressure. Purification of the crude by automated FC (Combiflash, EtOAc/heptane 0:100? 60:40) yields the title product. LC-MS: tR= 0.80 min, MH+= 257.17 (conditions 3). |
|
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; |
(3-methyloxetan-3-yl)methanol (CAS No: 3143-02-0, 2.0 ml_, 20 mmol) was dissolved in dichloromethane (30 mL) and cooled down to 0C. Triethylamine (3.0 ml_, 22 mmol), 4- dimethylaminopyridine (239 mg, 1.96 mmol) and 4-methylbenzene-1 -sulfonyl chloride (4.1 1 g, 21.5 mmol) were added, the ice bath removed, and the reaction stirred overnight at room temperature. The crude was then diluted with dicloromethane and treated with a 2M HCI solution. The organic pahse was separated and washed with sodium bicarbonate and brine, dried over sodium sulfate, filtered and evaporated. The crude product was used in the following reaction with no further purification. LC-MS (Method 1 ): Rt = 1 .06 min; MS (ESIpos): m/z = 257 [M+H]+ 1H-NMR (400 MHz, DMSO-d6) delta [ppm]: 1 .18 (s, 3H), 2.43 (s, 3H), 4.1 1 (s, 2H), 4.19 (d, 2H), 4.26 (d, 2H), 7.50 (d, 2H), 7.85 (d, 2H). |
|
With dmap; triethylamine; In dichloromethane; at 25℃; for 3h; |
Compound WX010-1 (200 mg, 1.96 mmol, 194.17 muL, 1 eq) and dichloromethane (5 mL) were added into a pre-dried 40 mL reaction vial, followed by sequential addition of triethylamine (297.24 mg, 2.94 mmol, 408.85 muL, 1.5 eq), dimethylaminopyridine (23.92 mg, 195.83 mumol, 0.1 eq) and p-toluenesulfonyl chloride (448.01 mg, 2.35 mmol, 1.2 eq). The reaction solution was stirred at 25C for 3 hours. A saturated aqueous solution of ammonium chloride (10 mL) was added to the reaction solution, and extracted with dichloromethane (10 mL×3). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give a crude product. The crude product was purified by thin layer chromatography silica gel plate (petroleum ether: ethyl acetate = 3:1) to give WX010-2. 1H NMR (400MHz, CHLOROFORM-d) delta = 7.82 (d, J=8.2 Hz, 2H), 7.37 (d, J=7.9 Hz, 2H), 4.44 - 4.30 (m, 4H), 4.12 (s, 2H), 2.47 (s, 3H), 1.32 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping