Alternatived Products of [ 1000787-76-7 ]
Product Details of [ 1000787-76-7 ]
| CAS No. : | 1000787-76-7 |
MDL No. : | MFCD19443922 |
| Formula : |
C17H11ClFN5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
339.75
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 1000787-76-7 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 1000787-76-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1000787-76-7 ]
- 1
-
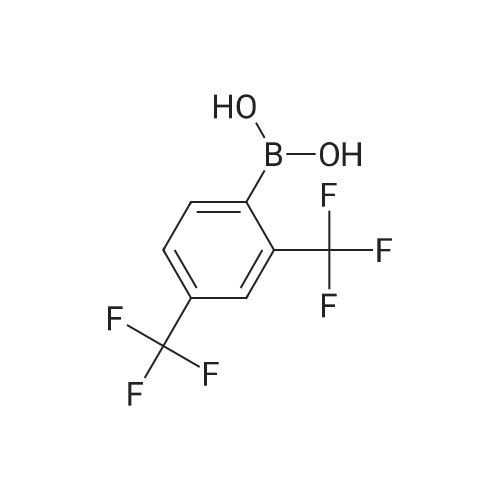 [ 153254-09-2 ]
[ 153254-09-2 ]

-
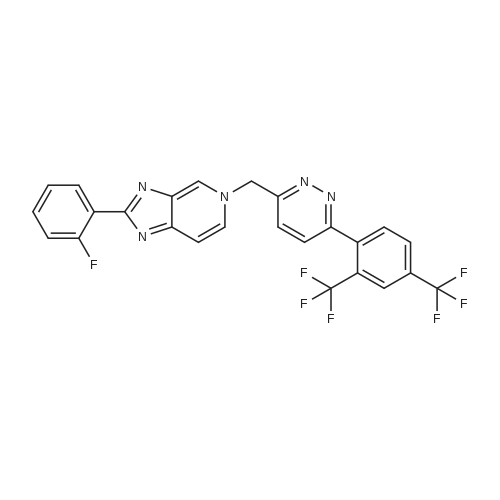
 [ 1000787-76-7 ]
[ 1000787-76-7 ]

-
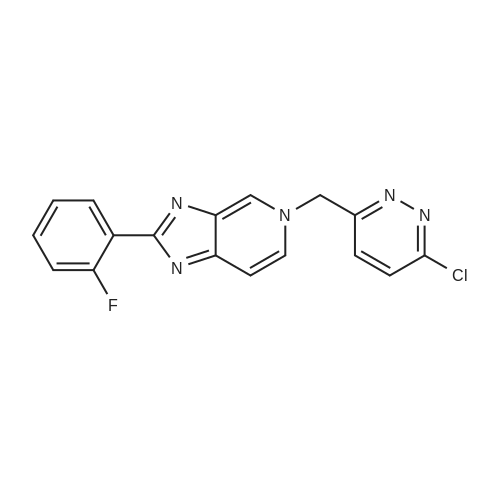
 [ 1000787-75-6 ]
[ 1000787-75-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In 1,2-dimethoxyethane; water; at 80℃; for 72h;Product distribution / selectivity; |
Compound A was dissolved in dimethoxyethane (DME). To this solution was added 2,4-bis(trifluromethyl)phenylboronic acid and a 2N aq. Na2CO3 solution. To the resulting biphasic mixture was added Pd(PPh3)4 and the reaction was then heated at 80 C. for 72 hrs. The reaction was cooled to room temperature and filtered through Celite and the Celite washed with EtOAc. The filtrate was concentrated in vacuo. The residue was purified on 6 g SiO2 using MeOH/CH2Cl2 to elute compound. The compound thus obtained was contaminated with PPh3(O). The product was repurified on a 1 mm Chromatotron plate with 0 to 5% MeOH/CH2Cl2 in 1% steps. The pure fractions were combined and concentrated in vacuo, then dried on high vacuum for 12 hrs. 11.8 mg of the free base of compound (1) was obtained with no PPh3 contamination. 1H NMR (300 MHz, CD3OD) 6.20 (s, 2) 7.32 (m, 3) 7.52 (m, 1) 7.78 (d, 1) 7.89 (d, 1) 7.95 (s, 2) 8.15 (m, 3) 8.35 (d, 1) 9.12 (s, 1) LC/MS M+H=518 |
| 11.8 mg |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In 1,2-dimethoxyethane; water; at 80℃; for 72h; |
Example 1 : 5-({6-[2,4-bis(trifluoromethyl)phenyl]pyridazin-3-yl}methyl)-2-(2-fluorophenyl)-5H- imidazo[4,5-c]pyridi Compound 103 was dissolved in dimethoxyethane (DME). To this solution was added 2,4-bis(trifluromethyl)phenylboronic acid 105 and a 2N aq. Na2C03 solution. To the resulting biphasic mixture was added Pd(PPh3)4 and the reaction was then heated at 80C for 72 hrs. The reaction was cooled to room temperature and filtered through Celite and the Celite washed with EtOAc. The filtrate was concentrated in vacuo. The residue was purified on 6g Si02 using MeOH/CH2CI2 to elute compound. The compound thus obtained was contaminated with PPh3(0). The product was repurified on a 1 mm Chromatotron plate with 0 to 5% MeOH/CH2CI2 in 1 % steps. The pure fractions were combined and concentrated in vacuo, then dried on high vacuum for 12 hrs. 11.8 mg of the free base of compound 1 was obtained with no PPh3 contamination. 1H NMR (300MHz,CD3OD) delta 6.20 (s, 2), 7.32 (m, 3), 7.52 (m, 1 ), 7.78 (d, 1), 7.89 (d, 1), 7.95 (s, 2), 8.15 (m, 3), 8.35 (d, 1), 9.12 (s, 1); LC/MS M+H = 518. |
- 2
-
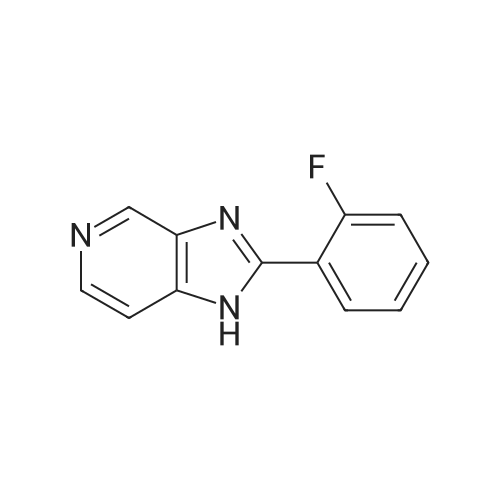 [ 89075-43-4 ]
[ 89075-43-4 ]

-
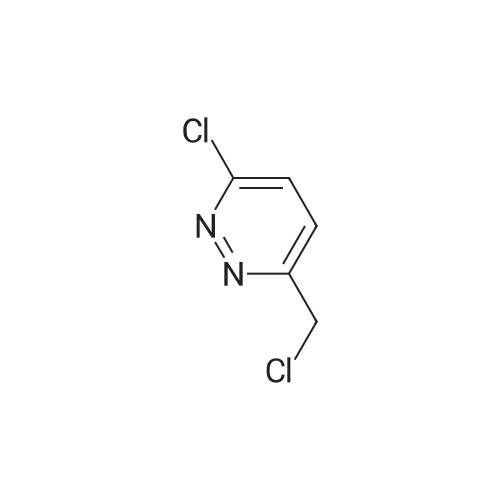 [ 120276-59-7 ]
[ 120276-59-7 ]

-
 [ 1000787-76-7 ]
[ 1000787-76-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydroxide; In N,N-dimethyl-formamide; for 3h; |
To a solution of core (obtained as described in literature in DMF (dimethylformamide), NaOH was added. Then SM for this step (obtained from step 1) was dissolved in DMF (20 ml) and added to the solution slowly. The reaction was stirred for 3 hrs, was diluted with water and extracted with EtOAc. The organic layer was dried with Na2SO4. The solvent was removed and the product recrystallized with DCM (dichloromethane). The yield was 5.7 g. |
| 5.7 g |
With sodium hydroxide; In N,N-dimethyl-formamide; for 3h; |
To a solution of compound 103 in DMF (dimethylformamide), NaOH was added. Compound 102 was dissolved in DMF (20 mL) and added to the solution slowly. The reaction was stirred for 3 hrs, was diluted with water and extracted with EtOAc. The organic layer was dried with Na2S0 . The solvent was removed and the product recrystallized with dichloromethane. The yield was 5.7 g of compound 103. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







