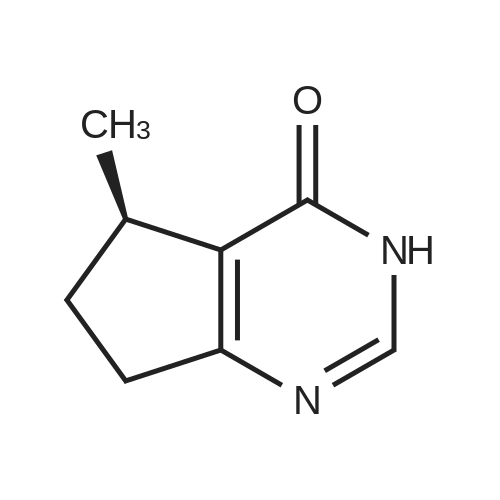
| 82.3% |
|
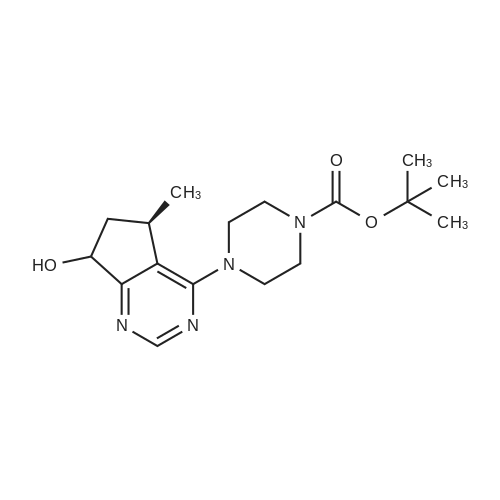
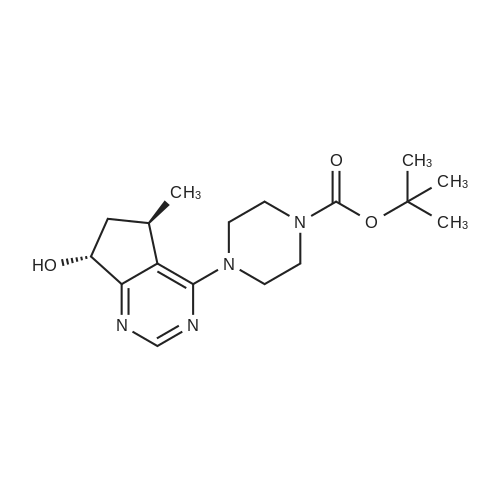
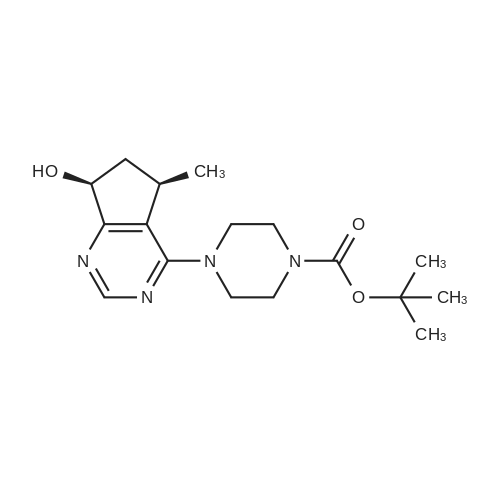
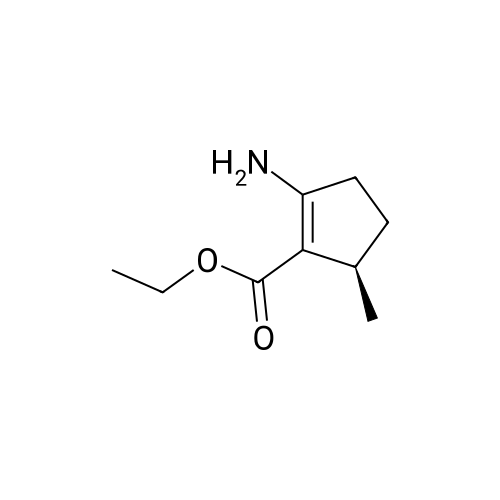
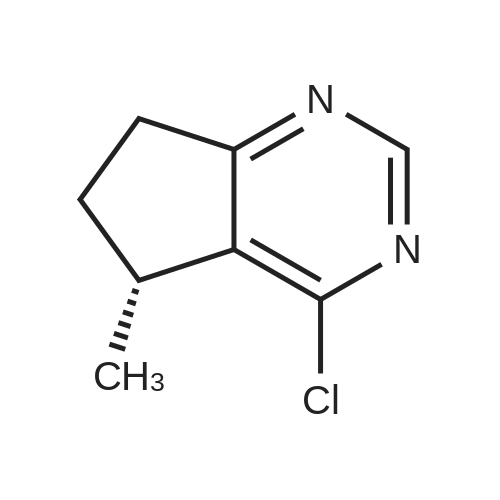
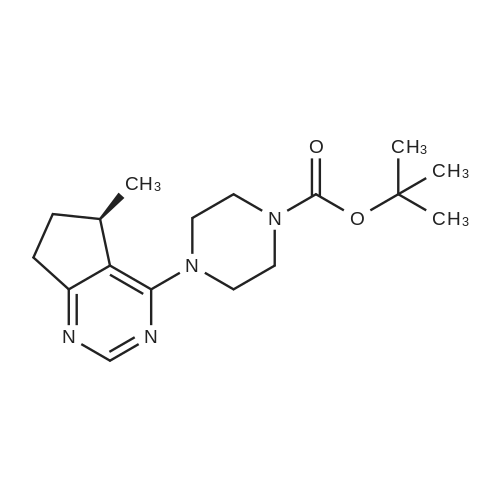
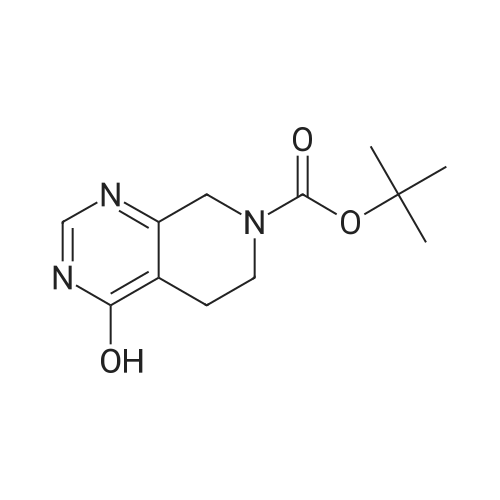
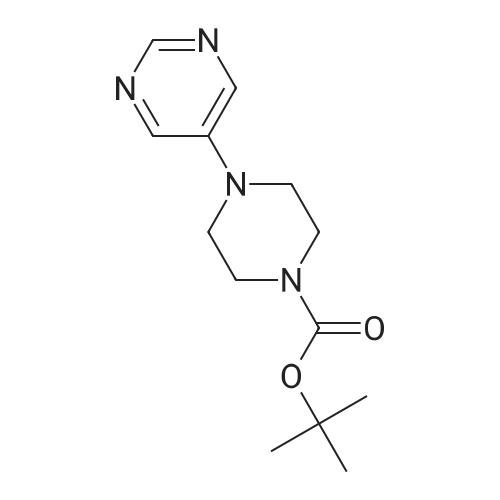
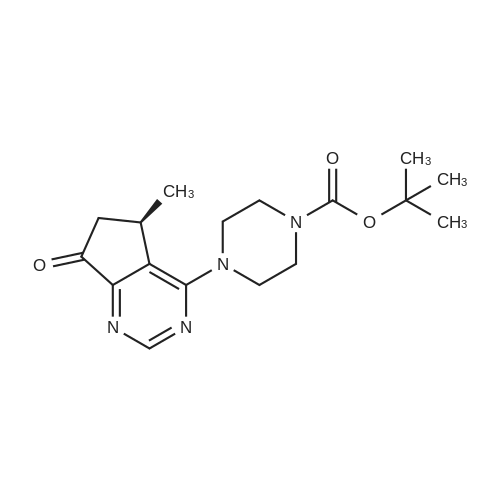
Step 8: A solution of DMSO (5.45 ml, 76.8 mmol) in DCM (50 mL) was added dropwise by addition funnel to a -78C solution of oxalyl chloride (3.35 ml, 38.4 mmol) in DCM (150 mL). The reaction mixture was stirred for 35 minutes, and then a solution of (5R)-tert-butyl 4-(7-hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4- yl)piperazine-l -carboxylate (9.17 g, 27.4 mmol) in DCM (80 mL) was added slowly by addition funnel. The reaction mixture was stirred another 1 hour at -78C, after which neat triethylamine (18.0 ml, 129 mmol) was added to the mixture. The reaction mixture was then allowed to warm to room temperature, and then it was stirred for 30 minutes. H20 was added. The mixture was extracted with DCM (3 X 200 mL), and the combined extracts were dried (Na2S04), filtered, and concentrated in vacuo. The crude was purified on silica gel (Biotage 65M): the column was flushed with ca. 800 mL 4: 1 DCM:EtOAc, then gradient to 1:1 DCM:ethyl acetate until product eluting, then 1 :4 DCM:EtOAc eluted product to give (R)-tert-butyl 4-(5-methyl-7-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 - carboxylate (7.5 g, 82.3% yield) as a brown foam. The foam was concentrated (3 X) from DCM/hexanes, which gave a very light brown foam. HPLC >95% area. LC/MS (APCI+) m/z 333 [M+H]+. |
| 82.3% |
|
Step 8: A solution of DMSO (5.45 ml, 76.8 mmol) in DCM (50 mL) was added dropwise by addition funnel to a -78C solution of oxalyl chloride (3.35 ml, 38.4 mmol) in DCM (150 mL). The reaction mixture was stirred for 35 minutes, and then a solution of (5R)-tert-butyl 4-(7-hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4- yl)piperazine- 1 -carboxylate (9.17 g, 27.4 mmol) in DCM (80 mL) was added slowly by addition funnel. The reaction mixture was stirred another 1 hour at -78C, after which neat triethylamine (18.0 ml, 129 mmol) was added to the mixture. The reaction mixture was then allowed to warm to room temperature, and then it was stirred for 30 minutes. H20 was added. The mixture was extracted with DCM (3 X 200 mL), and the combined extracts were dried (Na2S04), filtered, and concentrated in vacuo. The crude was purified on silica gel (Biotage 65M): the column was flushed with ca. 800 mL 4:1 DCM:EtOAc, then gradient to 1 : 1 DCM:ethyl acetate until product eluting, then 1 :4 DCM:EtOAc eluted product to give (R)-tert-butyl 4-(5-methyl-7-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 - carboxylate (7.5 g, 82.3% yield) as a brown foam. The foam was concentrated (3 X) from DCM/hexanes, which gave a very light brown foam. HPLC >95% area. LC/MS (APCI+) m/z 333 [M+H]+. |
| 82.3% |
|
Step 8:; A solution of DMSO (5.45 ml, 76.8 mmol) in DCM (50 mL) was added dropwise by addition funnel to a -780C solution of oxalyl chloride (3.35 ml, 38.4 mmol) in DCM (150 mL). The reaction mixture was stirred for 35 minutes, and then a solution of (5R)-tert-butyl 4- (7-hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine-l -carboxylate (9.17 g, 27.4 mmol) in DCM (80 mL) was added slowly by addition funnel. The reaction mixture was stirred another 1 hour at -78C, after which neat triethylamine (18.0 ml, 129 mmol) was added to the mixture. The reaction mixture was then allowed to warm to room temperature, and then it was stirred for 30 minutes. H2O was added. The mixture was extracted with DCM (3 X 200 mL), and the combined extracts were dried (Na2SO4), filtered, and concentrated in vacuo. The crude was purified on silica gel (Biotage 65M): the column was flushed with ca. 800 mL 4:1 DCM:EtOAc, then gradient to 1:1 DCM:ethyl acetate until product eluting, then 1:4 DCM:EtOAc eluted product to give (R)-tert-butyl 4-(5-methyl-7-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 - carboxylate (7.5 g, 82.3% yield) as a brown foam. The foam was concentrated (3 X) from DCM/hexanes, which gave a very light brown foam. HPLC >95% area. LC/MS (APCI+) m/z 333 [M+H]+. |
| 79.6 - 82.3% |
|
Step 3: To a -78 0C solution of oxalyl chloride (0.203 mL, 2.33 mmol) in 10 mLDCM was added dropwise by syringe a solution of DMSO (0.330 mL, 4.66 mmol) in 3 mL DCM. The reaction mixture was stirred 35 minutes, then a solution of (R)-tert-butyl 4-(7-hydroxy-5- methyl-^-dihydro-SH-cyclopentafdJpyrimidin^-y^piperazine-l-carboxylate (0.556 g, 1.66 mmol) in 5 mL DCM was added slowly by syringe. The reaction mixture was stirred another 1 hour at -78 0C, after which neat TEA (1.09 mL, 7.81 mmol) was added. The reaction mixture was then allowed to warm to room temperature, stirred 30 minutes, and H2O was added. The mixture was extracted with 3 x 75 mL DCM, and the combined extracts were dried (Na2SO4), filtered, and concentrated in vacuo. The crude was purified on silica gel (Biotage 40M): the column was flushed with about 300 mL 4:1 DCM:EtOAc, then gradient to 1:4 DCM: EtOAc to give (R)-tert- butyl 4-(5-methyl-7-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 -carboxylate (0.440 g, 79.6% yield) as a brown foam. MS (APCI+) m/z 333 [M+H]+. 1H NMR (CDCl3, 400 MHz) delta 8.73 (s, IH), 3.93-3.82 (m, 2H), 3.74-3.48 (m, 7H), 2.96 (dd, J = 19.6, 7.3 Hz, IH), 2.34 (dd, J= 19.6, 1.5 Hz, IH), 1.50 (s, 9H), 1.32 (d, J= 6.8 Hz, 3H).; Step 8: A solution of DMSO (5.45 mL, 76.8 mmol) in DCM (50 m) was added dropwise by addition funnel to a -78C solution of oxalyl chloride (3.35 mL, 38.4 mmol) in DCM (150 mL). The reaction mixture was stirred for 35 minutes. A solution of (5R)-tert-butyl 4-(7- hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 -carboxylate (9.17 g, 27.4 mmol) in DCM (80 mL) was added slowly by addition funnel. The reaction mixture was stirred for another 1 hour at -78C, after which neat NEt3 (18.0 mL, 129 mmol) was added. The reaction mixture was then allowed to warm to room temperature, stirred 30 minutes, and then H2O was added. The mixture was extracted with DCM (3 X 200 mL), and the combined extracts were dried (Na2SO4), filtered, and concentrated in vacuo. The crude was purified on silica gel (Biotage 65M): the column was flushed with ca. 800 mL 4:1 DCM:EtOAc, then gradient to 1:1 DCM:ethyl acetate until product eluting, then 1:4 DCM:EtOAc eluted product to give (R)-tert-butyl 4-(5- methyl-7-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-yl)piperazine- 1 -carboxylate (7.5 g, 82.3% yield) as a brown foam. The foam was concentrated (3 X) from DCM/hexanes, which gave a very light brown foam. HPLC >95% area. LC/MS (APCI+) m/z 333 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping