Alternatived Products of [ 100613-36-3 ]
Product Details of [ 100613-36-3 ]
| CAS No. : | 100613-36-3 |
MDL No. : | |
| Formula : |
C13H16O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
252.26
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 100613-36-3 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 100613-36-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100613-36-3 ]
- 1
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
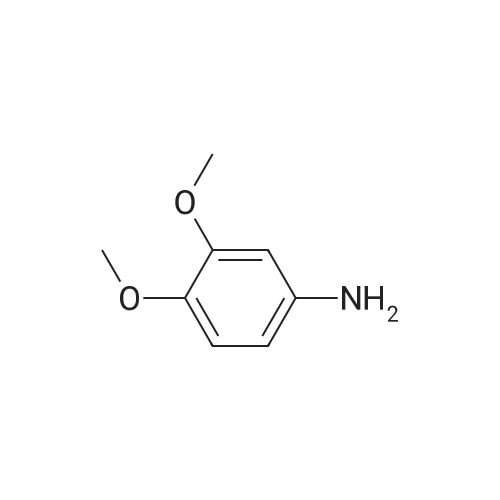 [ 6315-89-5 ]
[ 6315-89-5 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride |
|
- 2
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 344247-32-1 ]
[ 344247-32-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With bromine In chloroform for 24h; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
/BRN= 1978359/; |
|
- 4
-
 [ 1026546-09-7 ]
[ 1026546-09-7 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 64% |
With copper(ll) sulfate pentahydrate In methanol; water at 60℃; for 0.5h; |
4.4. General procedure for the preparation of a-aroylacetones 7b-e (Scheme 4a)
General procedure: To a solution of CuSO4*5H2O (2.0 equiv) in CH3OH/H2O (3:1, ca.3 mL per mmol of 10b-e) was added the propargylated a-arylmorpholinonitrile 10b-e (1.0 equiv). After 30 min stirring at 60°C,the solvents were evaporated before adding H2O and the resulting mixture was then extracted with CH2Cl2. to the residue was added water and the aqueous layer was extracted with dichloromethane. The combined organic phases were washed with brine, dried over Na2SO4, filtered and evaporated under reduced pressure. Purification of the residue by column chromatography, eluting with heptane/EtOAc, furnished the expected a-aroylacetones 7b-e in pure forms. |
|
With copper(II) sulfate In methanol; water at 60℃; for 1.5h; |
|
Reference:
[1]Chassaing, Stefan; Isorez-Mahler, Géraldine; Kueny-Stotz, Marie; Brouillard, Raymond
[Tetrahedron, 2015, vol. 71, # 20, p. 3066 - 3078]
[2]Mahalingam, Sakkarapalayam M.; Aidhen, Indrapal Singh
[Journal of Organic Chemistry, 2006, vol. 71, # 1, p. 349 - 351]
- 5
-
 [ 68415-05-4 ]
[ 68415-05-4 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: NaH / dimethylformamide / 1 h / 27 - 30 °C
2: CuSO4*5H2O / methanol; H2O / 1.5 h / 60 °C |
|
|
Multi-step reaction with 2 steps
1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 20 °C
1.2: 2 h / 20 °C
2.1: copper(ll) sulfate pentahydrate / methanol; water / 0.5 h / 60 °C |
|
Reference:
[1]Mahalingam, Sakkarapalayam M.; Aidhen, Indrapal Singh
[Journal of Organic Chemistry, 2006, vol. 71, # 1, p. 349 - 351]
[2]Chassaing, Stefan; Isorez-Mahler, Géraldine; Kueny-Stotz, Marie; Brouillard, Raymond
[Tetrahedron, 2015, vol. 71, # 20, p. 3066 - 3078]
- 6
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: bromine / CHCl3 / 24 h
2: acetone / 24 h
3: 60 percent / ammonium hydroxyde / H2O; CHCl3 |
|
- 7
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 107504-59-6 ]
[ 107504-59-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: bromine / CHCl3 / 24 h
2: acetone / 24 h |
|
- 8
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 7473-34-9 ]
[ 7473-34-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane
4: aqueous H2O2; acetone
5: 240 °C |
|
|
Multi-step reaction with 4 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane
4: 240 °C |
|
- 9
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 5465-02-1 ]
[ 5465-02-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: aqueous HCl
2: sulfuric acid |
|
- 10
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 7473-33-8 ]
[ 7473-33-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 6 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane
4: aqueous H2O2; acetone
5: 240 °C
6: sodium; ethanol |
|
|
Multi-step reaction with 5 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane
4: 240 °C
5: sodium; ethanol |
|
- 11
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 102659-44-9 ]
[ 102659-44-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane |
|
- 12
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 102659-48-3 ]
[ 102659-48-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane
4: aqueous H2O2; acetone |
|
|
Multi-step reaction with 3 steps
1: aqueous HCl
2: sulfuric acid
3: SeO2; aqueous dioxane |
|
- 13
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 106-93-4 ]
[ 106-93-4 ]

-
 [ 1178542-11-4 ]
[ 1178542-11-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
Stage #1: 1-(3,4,5-trimethoxyphenyl)butane-1,3-dione With potassium carbonate In dimethyl sulfoxide at 20℃;
Stage #2: ethylene dibromide In dimethyl sulfoxide at 20℃; |
|
- 14
-
 [ 4521-61-3 ]
[ 4521-61-3 ]

-
 [ 67-64-1 ]
[ 67-64-1 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With lithium diisopropyl amide In tetrahydrofuran at -78 - 20℃; |
|
- 15
-
 [ 86-81-7 ]
[ 86-81-7 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: methanol; water / 4 h / 30 °C
2.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 20 °C
2.2: 2 h / 20 °C
3.1: copper(ll) sulfate pentahydrate / methanol; water / 0.5 h / 60 °C |
|
- 16
-
 [ 108-73-6 ]
[ 108-73-6 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
| 97% |
With hexafluorophosphoric acid; acetic acid In water at 20℃; |
4.6. General procedure for the preparation of 4-methylflavylium salts 6a-c,e,h (Schemes 3 and 5), 15 (Table 1)
General procedure: An excess of aqueous HPF6 (0.5 mL per mmol of phenol, 50% inw ater) was added to a solution of phenol (1.0 equiv), i.e. phloroglucinol dihydrate 8 or resorcinol, and a-aroylacetone 7a-e,h (1.0 equiv) in a minimum of AcOH (ca. 2 mL per mmol of phenol). The solution, becoming immediately dark red, was stirred at room temperature for up to 48 h. The resulting mixture was then plunged into Et2O where the 4-methylflavylium hexafluorophosphate precipitated. The so-obtained precipitate was recovered by filtration, washed with Et2O and finally dried under vacuum to give the expected 4-methylflavylium salts 6a-f,h in pure forms. |
- 17
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: acetic acid; hexafluorophosphoric acid / water / 20 °C
2: ethanol / 24 h / Reflux |
|
- 18
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ CAS Unavailable ]
[ CAS Unavailable ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: acetic acid; hexafluorophosphoric acid / water / 20 °C
2: ethanol / 24 h / Reflux |
|
- 19
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 59-88-1 ]
[ 59-88-1 ]

-
 [ 1817608-99-3 ]
[ 1817608-99-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 74% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 20
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
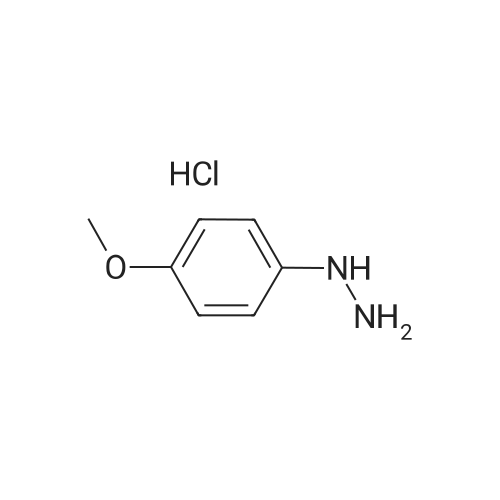 [ 19501-58-7 ]
[ 19501-58-7 ]

-
 [ 1819961-92-6 ]
[ 1819961-92-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 78% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 21
-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
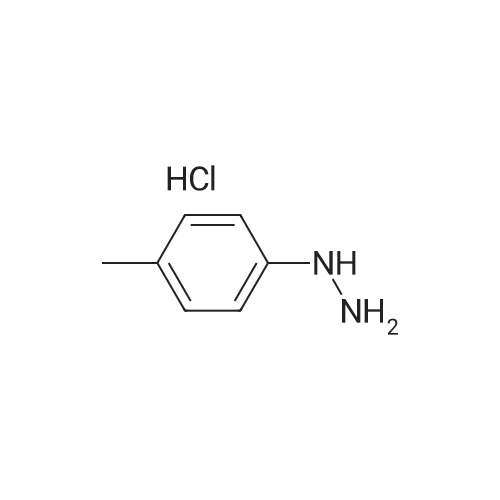 [ 637-60-5 ]
[ 637-60-5 ]

-
 [ 1819961-91-5 ]
[ 1819961-91-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 73% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 22
-
 [ 1049739-45-8 ]
[ 1049739-45-8 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-97-1 ]
[ 1819961-97-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 71% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 23
-
 [ 1049739-45-8 ]
[ 1049739-45-8 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-98-2 ]
[ 1819961-98-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: sodium acetate / ethanol / 1 h / Reflux
2.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.17 h / 65 °C
2.2: 1.5 h / 65 °C |
|
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 24
-
 [ 1819962-02-1 ]
[ 1819962-02-1 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-93-7 ]
[ 1819961-93-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 78% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 25
-
 [ 1819962-02-1 ]
[ 1819962-02-1 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-94-8 ]
[ 1819961-94-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: sodium acetate / ethanol / 1 h / Reflux
2.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.17 h / 65 °C
2.2: 1.5 h / 65 °C |
|
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 26
-
 [ 1819962-04-3 ]
[ 1819962-04-3 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-95-9 ]
[ 1819961-95-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 77% |
With sodium acetate In ethanol for 1h; Reflux; |
3-Methyl-5-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-pyrazole (7a)
General procedure: (3,4,5-Trimethoxyphenyl)hydrazine hydrochloride (0.14 g, 0.617 mmol), 1-benzoylacetone (0.10 g, 0.617 mmol) and NaOAc (0.05 g, 0.074 mmol) were combined in EtOH (50 mL). The reaction was refluxedfor 1 h, cooled to room temperature concentrated in vacuo. The resulting slurry was dissolved in dichloromethane (50 mL), washed with water (2 × 30 mL), dried over Na2SO4, filtered and concentrated under vacuum. The crude product was purified by chromatography on silica gel to afford the desired 5-phenyl-1-(3,4,5-trimethoxyphenyl)- 3-methyl-1H-pyrazole 7a. |
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]
- 27
-
 [ 1819962-04-3 ]
[ 1819962-04-3 ]

-
 [ 100613-36-3 ]
[ 100613-36-3 ]

-
 [ 1819961-96-0 ]
[ 1819961-96-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: sodium acetate / ethanol / 1 h / Reflux
2: titanium tetrachloride / dichloromethane / 0.5 h / -20 °C |
|
Reference:
[1]Xu, Qile; Qi, Huan; Sun, Maolin; Zuo, Daiying; Jiang, Xuewei; Wen, Zhiyong; Wang, Zhiwei; Wu, Yingliang; Zhang, Weige
[PLoS ONE, 2015, vol. 10, # 6]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








