Alternatived Products of [ 101268-55-7 ]
Product Details of [ 101268-55-7 ]
| CAS No. : | 101268-55-7 |
MDL No. : | MFCD00845709 |
| Formula : |
C13H18O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
238.28
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 101268-55-7 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 101268-55-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 101268-55-7 ]
- 1
-
 [ 2049-80-1 ]
[ 2049-80-1 ]

-
 [ 106-96-7 ]
[ 106-96-7 ]

-
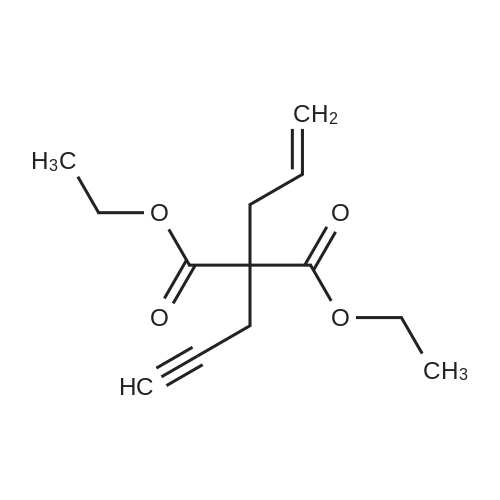 [ 101268-55-7 ]
[ 101268-55-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 79% |
|
To a suspension of sodium hydride (360 mg, 15.0 mmol) indry THF (20 mL), compound 13a (1 g, 5.0 mmol) was added and the reaction mixture wasstirred at r.t. for 15 min. Later, propargyl bromide (1.40 mL, 15 mmol) was added and thestirring was continued for 16 h at same temperature. At the conclusion of the reaction (TLCmonitoring), the reaction mixture was diluted with EtOAc and the aqueous layer wasextracted with CH2Cl2. Then, the solvent was removed under reduced pressure and the crudeproduct was purified by silica gel column chromatography (3% EtOAc-petroleum ether) toafford the compound 15a as a yellow liquid (943 mg, 79%). The 1H and 13C NMR spectra matched with the literature reported spectral data. |
| 79% |
|
To a suspension of sodium hydride (360 mg, 15.0 mmol) in dry THF (20 mL), compound 13a (1 g, 5.0 mmol) was added and the reaction mixture was stirred at r.t. for 15 min. Later, propargyl bromide (1.40 mL, 15 mmol) was added and the stirring was continued for 16 h at same temperature. At the conclusion of the reaction (TLC monitoring), the reaction mixture was diluted with EtOAc and the aqueous layer was extracted with CH2Cl2. Then, the solvent was removed under reduced pressure and the crude product was purified by silica gel column chromatography (3% EtOAc-petroleum ether) to afford the compound 15a as a yellow liquid (943 mg, 79%). The 1H and 13C NMR spectra matched with the literature reported spectral data |
|
|
Cap-12: 5,5-difluorooctahydropentalene-2-carboxylic acid NaH (60%, 0.480 g) was added to a cold (0 C.) solution of diethyl 2-allylmalonate (2.002 g) in DMF (10 mL) and the mixture was allowed to warm to rt over 30 min. Then a solution of 3-bromoprop-1-yne (1.487 g) in DMF (3 mL) was added dropwise at 0 C. and the mixture was allowed to warm to rt and stirred at rt overnight. The reaction mixture was diluted with ether and then quenched with sat. NH4Cl, washed with water, brine and dried (Na2SO4). The crude product was purified by silica gel FCC (1:1 DCM-hexane) to afford diethyl 2-allyl-2-(prop-2-ynyl)malonate as a clear oil. |
|
|
NaH (60%, 0.480 g) was added to a cold (0 C) solution of diethyl 2- allylmalonate (2.002 g) in DMF (10 mL) and the mixture was allowed to warm to rt over 30 min. Then a solution of 3-bromoprop-l-yne (1.487 g) in DMF (3 mL) was added dropwise at 0 C and the mixture was allowed to warm to rt and stirred at rt overnight. The reaction mixture was diluted with ether and then quenched with sat. NH4C1, washed with water, brine and dried ( a2S04). The crude product was purified by silica gel FCC (1 : 1 DCM-hexane) to afford diethyl 2-allyl-2-(prop-2- ynyl)malonate as a clear oil. |

Reference:
[1]Tetrahedron Letters,2006,vol. 47,p. 7389 - 7393
[2]Journal of the American Chemical Society,2009,vol. 131,p. 8772 - 8774
[3]Synthesis,2013,vol. 45,p. 174 - 182
[4]Organic Letters,2013,vol. 15,p. 2398 - 2401
[5]Journal of the American Chemical Society,2010,vol. 132,p. 13214 - 13216
[6]European Journal of Organic Chemistry,2020,vol. 2020,p. 609 - 617
[7]Chemistry - An Asian Journal,2021,vol. 16,p. 3909 - 3913
[8]Journal of the American Chemical Society,2002,vol. 124,p. 9164 - 9174
[9]Advanced Synthesis and Catalysis,2011,vol. 353,p. 1865 - 1870
[10]Synthesis,2014,vol. 46,p. 2471 - 2480
[11]Synthesis,2014,vol. 46,p. 2471 - 2480
[12]Journal of Organometallic Chemistry,2001,vol. 624,p. 73 - 87
[13]European Journal of Organic Chemistry,2011,p. 1557 - 1569
[14]Journal of the American Chemical Society,1994,vol. 116,p. 8593 - 8601
[15]Journal of the American Chemical Society,1999,vol. 121,p. 1245 - 1255
[16]Journal of the American Chemical Society,2002,vol. 124,p. 3806 - 3807
[17]Angewandte Chemie - International Edition,2012,vol. 51,p. 10861 - 10865
Angew. Chem.,2012,vol. 124,p. 11019 - 11023
[18]Patent: US2013/183269,2013,A1 .Location in patent: Paragraph 1178; 1179
[19]Patent: WO2015/5901,2015,A1 .Location in patent: Page/Page column 510
- 2
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
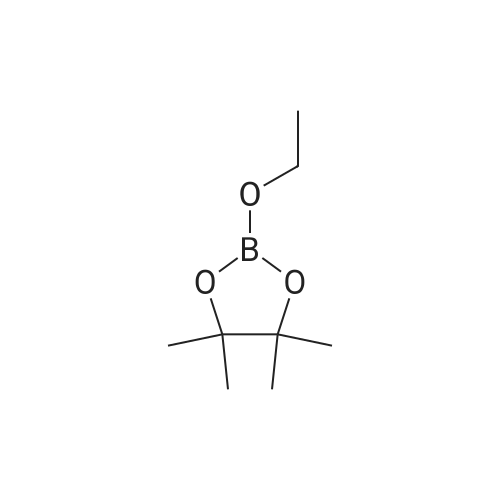 [ 1126-93-8 ]
[ 1126-93-8 ]

-
2-allyl-2-[3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-prop-2-ynyl]-malonic acid diethyl ester
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: 4,4-bis(ethoxycarbonyl)-1-hepten-6-yne With lithium diisopropyl amide In diethyl ether at -78℃;
Stage #2: 2-ethoxy-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane In diethyl ether
Stage #3: With hydrogenchloride In diethyl ether at -78 - 20℃; |
|
- 3
-
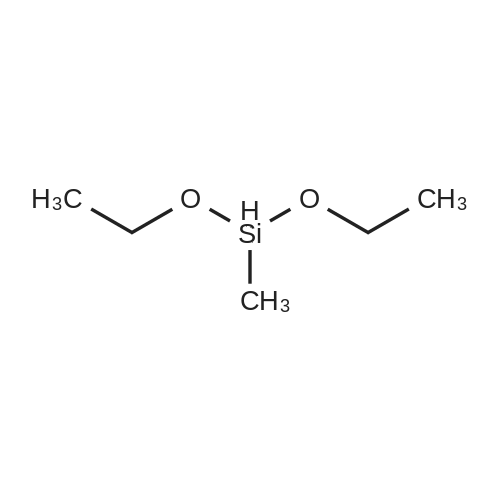 [ 2031-62-1 ]
[ 2031-62-1 ]

-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
4,4-bis(carbethoxy)-1-(Z)-methyldiethoxysilylmethylidene-2-methylcyclopentane
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With carbon monoxide In hexane at 20℃; for 0.0833333h; |
|
Reference:
[1]Ojima, Iwao; Vu, An T.; Lee, Seung-Yub; McCullagh, James V.; Moralee, Andrew C.; Fujiwara, Masaki; Hoang, Tram H.
[Journal of the American Chemical Society, 2002, vol. 124, # 31, p. 9164 - 9174]
- 4
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
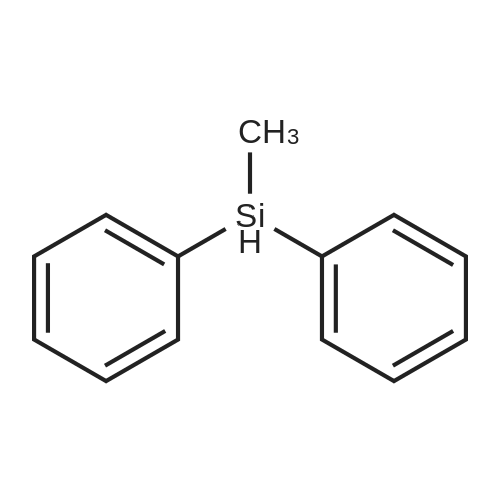 [ 776-76-1 ]
[ 776-76-1 ]

-
4,4-bis(carbethoxy)-1-(Z)-methyldiphenylsilylmethylidene-2-methylcyclopentane
[ No CAS ]
- 5
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
 [ 201230-82-2 ]
[ 201230-82-2 ]

-
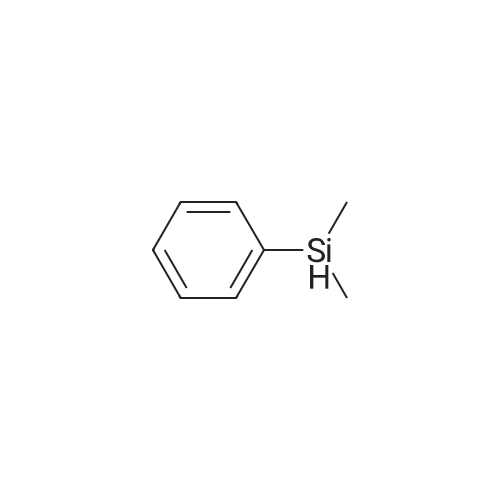 [ 766-77-8 ]
[ 766-77-8 ]

-
4,4-bis(carbethoxy)-1(Z)-dimethylphenylsilylmethylidene-2-formylmethylcyclopentane
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 96% |
In 1,4-dioxane at 105℃; for 12h; |
|
| 91% |
With triethyl phosphite In 1,4-dioxane at 105℃; for 48h; |
|
Reference:
[1]Park, Kang Hyun; Jung, Il Gu; Kim, So Yeon; Chung, Young Keun
[Organic Letters, 2003, vol. 5, # 26, p. 4967 - 4970]
[2]Ojima, Iwao; Vu, An T.; Lee, Seung-Yub; McCullagh, James V.; Moralee, Andrew C.; Fujiwara, Masaki; Hoang, Tram H.
[Journal of the American Chemical Society, 2002, vol. 124, # 31, p. 9164 - 9174]
- 6
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
 [ 766-77-8 ]
[ 766-77-8 ]

-
4,4-bis(carbethoxy)-1-(Z)-dimethylphenylsilylmethylidene-2-methylcyclopentane
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 97% |
In hexane at 22℃; for 2h; |
|
| 95% |
With carbon monoxide In hexane at 20℃; for 0.0833333h; |
|
Reference:
[1]Park, Kang Hyun; Jung, Il Gu; Kim, So Yeon; Chung, Young Keun
[Organic Letters, 2003, vol. 5, # 26, p. 4967 - 4970]
[2]Ojima, Iwao; Vu, An T.; Lee, Seung-Yub; McCullagh, James V.; Moralee, Andrew C.; Fujiwara, Masaki; Hoang, Tram H.
[Journal of the American Chemical Society, 2002, vol. 124, # 31, p. 9164 - 9174]
- 7
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
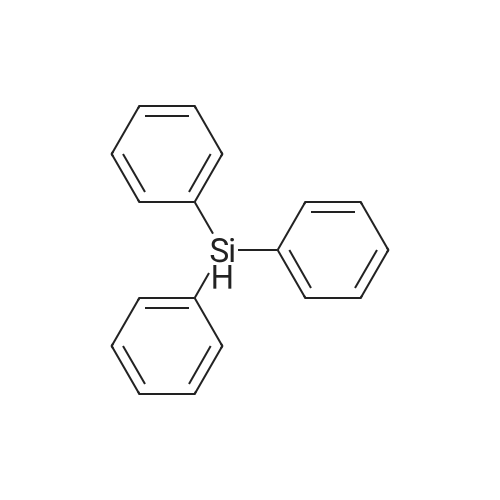 [ 789-25-3 ]
[ 789-25-3 ]

-
4,4-bis(carbethoxy)-1-(Z)-triphenylsilylmethylidene-2-methylcyclopentane
[ No CAS ]
- 8
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
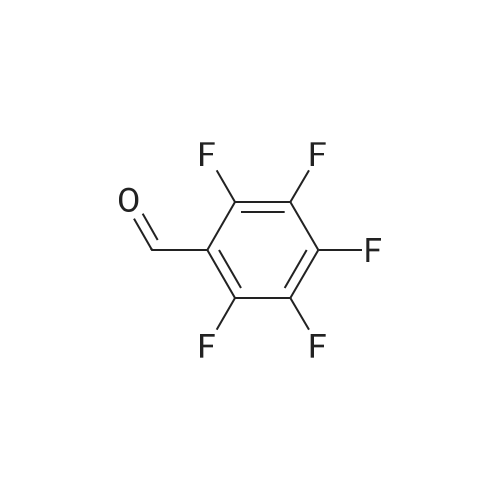 [ 653-37-2 ]
[ 653-37-2 ]

-
7,7-bis(ethoxycarbonyl)bicyclo[3.3.0]-1-octene-3-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 71% |
With 1,3-bis-(diphenylphosphino)propane In xylene at 130℃; for 24h; |
|
- 9
-
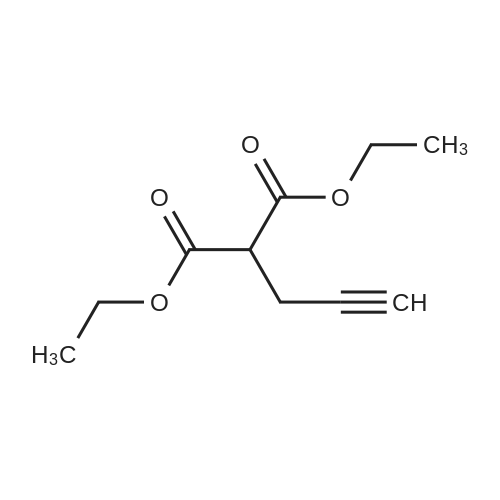 [ 17920-23-9 ]
[ 17920-23-9 ]

-
 [ 106-95-6 ]
[ 106-95-6 ]

-
 [ 101268-55-7 ]
[ 101268-55-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 97% |
Stage #1: diethyl propargylmalonate With sodium hydride In tetrahydrofuran at 0 - 20℃; Inert atmosphere;
Stage #2: allyl bromide In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; |
|
| 90% |
Stage #1: diethyl propargylmalonate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 2h;
Stage #2: allyl bromide In tetrahydrofuran; mineral oil at 0 - 20℃; for 48h; |
|
| 66% |
Stage #1: diethyl propargylmalonate With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide; mineral oil at 0℃; for 1h; Inert atmosphere;
Stage #2: allyl bromide With sodium iodide In tetrahydrofuran; N,N-dimethyl-formamide; mineral oil at 20℃; |
|
| 41% |
Stage #1: diethyl propargylmalonate With sodium ethanolate In N,N-dimethyl-formamide; toluene
Stage #2: allyl bromide In N,N-dimethyl-formamide; toluene at 80℃; for 14h; |
|

Reference:
[1]Benedetti, Erica; Simonneau, Antoine; Hours, Alexandra; Amouri, Hani; Penoni, Andrea; Palmisano, Giovanni; Malacria, Max; Goddard, Jean-Philippe; Fensterbank, Louis
[Advanced Synthesis and Catalysis, 2011, vol. 353, # 11-12, p. 1908 - 1912]
[2]Mandal, Joydeb; Krishna Prasad; Rao, D. S. Shankar; Ramakrishnan
[Journal of the American Chemical Society, 2014, vol. 136, # 6, p. 2538 - 2545]
[3]Gao, Ming; Gao, Qiangqiang; Hao, Xiangbin; Wu, Ying; Zhang, Qingmin; Liu, Guohua; Liu, Rui
[Organic Letters, 2020, vol. 22, # 3, p. 1139 - 1143]
[4]Shimamoto, Takamitsu; Chimori, Motoharu; Sogawa, Hiroaki; Yamamoto, Keiji
[Journal of the American Chemical Society, 2005, vol. 127, # 47, p. 16410 - 16411]
- 10
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
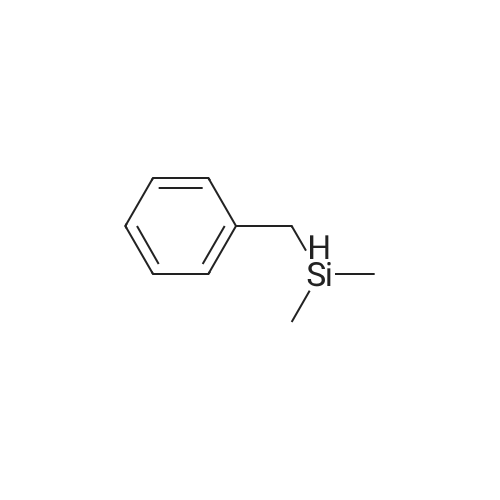 [ 1631-70-5 ]
[ 1631-70-5 ]

-
 [ 935471-51-5 ]
[ 935471-51-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 84% |
With dodecacarbonyltetrarhodium(0); carbon monoxide In hexane at 20℃; for 0.166667h; |
|
- 11
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
 [ 201230-82-2 ]
[ 201230-82-2 ]

-
 [ 1631-70-5 ]
[ 1631-70-5 ]

-
diethyl (Z)-3-benzyldimethylsilylmethylene-4-oxoethylcyclopentane-1,1-dicarboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 71% |
With dodecacarbonyltetrarhodium(0); triethyl phosphite In 1,4-dioxane at 105℃; for 48h; |
|
- 12
-
 [ 101268-55-7 ]
[ 101268-55-7 ]

-
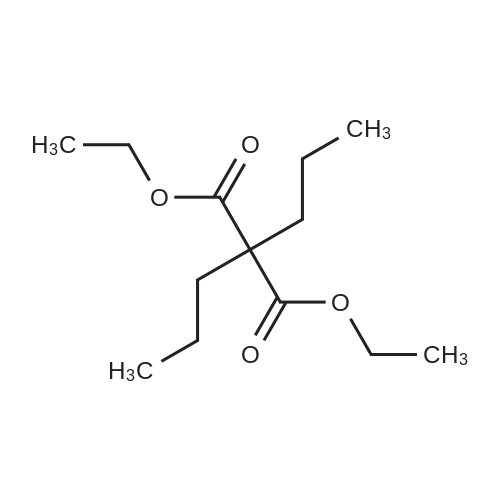 [ 6065-63-0 ]
[ 6065-63-0 ]

-
 [ 1114376-78-1 ]
[ 1114376-78-1 ]

-
diethyl (3R,4R)-3,4-dimethylcyclopentane-1,1-dicarboxylate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 25.9 %Chromat. |
With (<SUP>IPr</SUP>2,6-(2,6-iPr<SUB>2</SUB>C<SUB>6</SUB>H<SUB>3</SUB>N=CMe)<SUB>2</SUB>C<SUB>5</SUB>H<SUB>3</SUB>N)Fe(N<SUB>2</SUB>)<SUB>2</SUB>; hydrogen In benzene at 23℃; for 3h; Inert atmosphere; optical yield given as %de; |
|
- 13
-
 [ 17920-23-9 ]
[ 17920-23-9 ]

-
 [ 106-96-7 ]
[ 106-96-7 ]

-
 [ 101268-55-7 ]
[ 101268-55-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 96% |
Stage #1: diethyl propargylmalonate With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Inert atmosphere;
Stage #2: propargyl bromide at 0 - 20℃; for 20.5h; Inert atmosphere; |
|
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping















