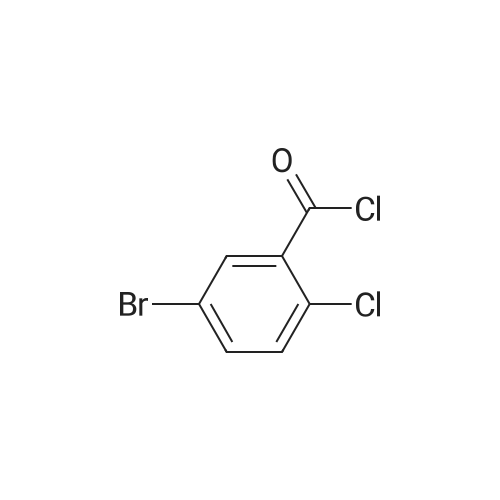
| 97% |
With sodium methylate In methanol at 45℃; for 4h; Industrial scale; |
|
| 95% |
With sodium methylate In methanol at 20℃; for 2h; |
6.8 6.8.
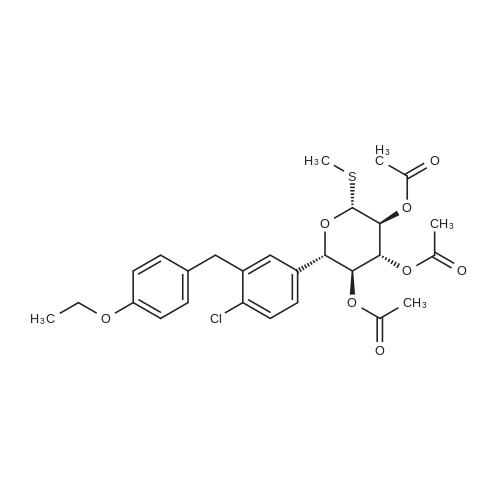
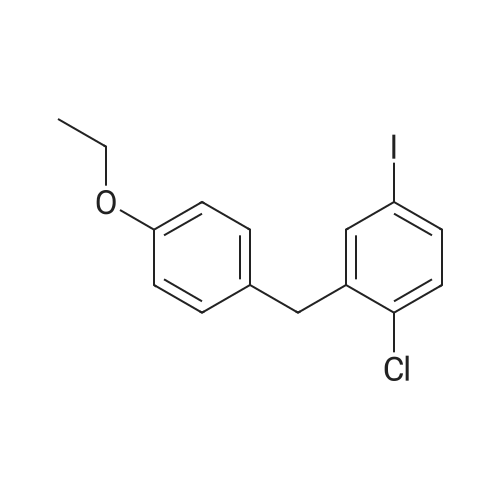
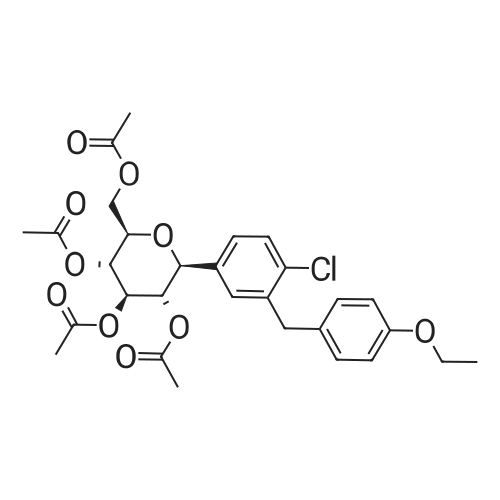
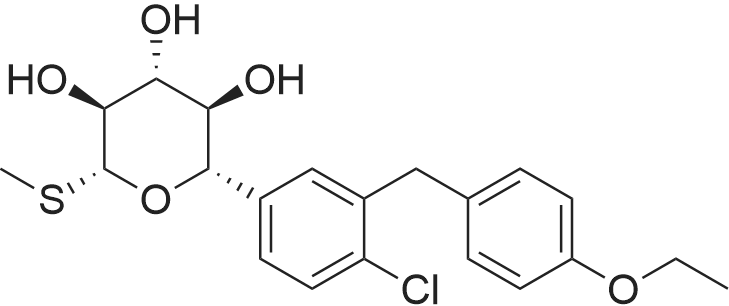
To a slurry of (2S,3S,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate (90.0 g, 0. 164 mol) in MeOH (900 mL, 10×) was added NaOMe in MeOH (25 wt %, 18 mL, 0.2×) at 20° C. and the mixture was stirred at 20° C. for 2 hours until all solids disappeared. The mixture was then concentrated to 300 mL, added to H2O (1 L) and stirred for 1 hour. The solid was filtered and washed with H2O (100 mL, ×3) and the cake was dried under vacuum at 45° C. overnight to afford the desired methyl thiolate (67.0 g, 95%). 1H NMR (CDCl3) 6 7.38 (d, J=8.4 Hz, 1H), 7.22 (m, 2H), 7.11 (d, J=8.8 Hz, 2H), 6.83 (d, J=8.8 Hz, 2H), 4.35 (d, J=9.6 Hz, 1H), 4.15 (d, J=9.6 Hz, 1H), 4.10-3.95 (m, 3H), 3.64 (t, J=8.8 Hz, 1H), 3.50 (m, 2H), 2.73 (br s, 3H), 2.17 (s, 3H), 1.40 (t, J=7.2 Hz, 3H). |
| 95% |
Stage #1: [(2S,3S,4R,5S,6R)-4,5-diacetoxy-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-methylthiotetrahydropyran-3-yl]acetate With sodium methylate In methanol at 20℃; for 2h;
Stage #2: With water In methanol for 1h; |
6.8
To a slurry of (2S,3S,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate (90.0 g, 0.164 mol) in MeOH (900 mL, 10×) was added NaOMe in MeOH (25 wt %, 18 mL, 0.2×) at 20° C. and the mixture was stirred at 20° C. for 2 hours until all solids disappeared. The mixture was then concentrated to 300 mL, added to H2O (1 L) and stirred for 1 hour. The solid was filtered and washed with H2O (100 mL, ×3) and the cake was dried under vacuum at 45° C. overnight to afford the desired methyl thiolate (67.0 g, 95%). 1H NMR (CDCl3) δ 7.38 (d, J=8.4 Hz, 1H), 7.22 (m, 2H), 7.11 (d, J=8.8 Hz, 2H), 6.83 (d, J=8.8 Hz, 2H), 4.35 (d, J=9.6 Hz, 1H), 4.15 (d, J=9.6 Hz, 1H), 4.10-3.95 (m, 3H), 3.64 (t, J=8.8 Hz, 1H), 3.50 (m, 2H), 3.42 (br s, 1H), 2.95 (br s, 1H), 2.57 (br s, 1H), 2.17 (s, 3H), 1.40 (t, J=7.2 Hz, 3H). |
| 93% |
With methanol; ammonia In dichloromethane at 26℃; for 14h; Inert atmosphere; |
8 (8) Preparation and data of compound 7a:
To a solution of columns Soxhlet net acetate 6a (17mg, 0.031mmol, 1equiv) in methanol / dichloromethane (3mL, v / v = 2: 1) was added a saturated ammonia / methanol solution (2mL), the reaction was stirred for 14 hours at 26°C , concentrated under reduced pressure, column chromatography (dichloromethane / methanol = 15: 1) to give the net Soxhlet column (12.2mg, 93%). |
| 93% |
With methanol; ammonia In dichloromethane at 20℃; for 2h; Inert atmosphere; |
|
| 86% |
With sodium methylate In methanol at 20℃; for 2h; Industry scale; |
6.11
To a 250 L reactor was charged the triacetate (10 kg) and methanol (75 kg). Sodium methoxide (1.6 kg, 30% solution) was added with 5 kg methanol rinse. The mixture was stirred at room temperature for at least 2 hours or until the reaction was complete. Charcoal (Darco G-60, 1 kg) was added with 5 kg methanol rinse. This mixture was heated at 40° C. for 1 h, cooled to room temperature, and filtered through celite. The cake was washed with methanol (10 kg). Water (100 kg) was added and the mixture was concentrated under vacuum. MTBE (200 kg) and water (50 kg) were added and phases were split. The organic layer was washed with water (100 kg) and concentrated under vacuum. MEK (100 kg) was added and the same about of solvent was distilled under vacuum. This MEK addition and distillation was repeated to dry the solution. Enough MEK was added to produce a solution of (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2H-pyran-3,4,5-triol in 50 L MEK. This solution was polish filtered and heptane (100 L) was added at about 80° C. Form 2 seeds (0.1 kg) were added followed by slow addition of heptane (100 L) as 80° C. Heating was continued for 8 h more at 80° C., cooled to 20° C. over at least 3 hours, held at this temperature for at least 2 hours, filtered, and washed with MEK/heptane. The cake was dried at 50° C. under vacuum to afford the title compound as a white solid (6.6 kg, 86% yield). |
| 77.8% |
With methanol; sodium methylate at 15 - 20℃; for 2h; |
06 Synthesis of Sot13
0.1 g of Sot12 was added to a 100 mL single-necked flask, 2 mL of methanol was added, and 0.25 mL of a 25% sodium methoxide methanol solution was added dropwise at 15-20 ° C, and the reaction was performed for 2 h. The reaction endpoint was monitored by HPLC. After the reaction was completed, the reaction solution was concentrated under reduced pressure at 40 ° C, 5 mL of methyl tert-butyl ether and 2 mL of water were added, and the mixture was separated. The organic phase was collected. The organic phase was concentrated under reduced pressure at 40 ° C to obtain the crude Sot13. Chromatographic purification (200-300 mesh silica gel, gradient elution polarity: ethyl acetate) gave 60 mg of a white solid, yield: 77.8%. |
|
With sodium methylate In methanol at 20℃; for 2h; |
16 Example-16: Preparation of Sotagliflozin Form 2:
[0096] 50 gm of (2S,3S,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6- (Methylthio) tetrahydro-2H-pyran-3,4,5-triyl triacetate (SOT-1) and 500ml of methanol were charged in round bottom flask, the slurry was cooled to 20°C then added sodium methoxide solution prepared in methanol (2.45gm of sodium methoxide in 50ml of methanol) at 20°C over the period of 10 min and stirred the mass for 2hr at 20°C.The reaction completion was ensured by HPLC. Once the reaction is completed added 2.5gm Norit carbon to the reaction mass at 23°C and stirred for 30min. Filtered the reaction mass through Hyflo bed and washed with 20ml of methanol. Taken the filtrate into the flask and concentrated under vacuum at 45°C up to 3 volumes with respect to SOT-1 then cooled to 21°C over the period of 60 min, added 560ml of Water at 21°C over the period of 30min and stirred for 30min at 21 °C, the reaction mass left overnight (without stirring) and stirred for lhr. The obtained slurry was filtered under vacuum and washed with 55ml*3times of water then kept for suction at 20- 30°C for 30min. The material was dried at 50-60°C for 9hrs under vacuum to obtain 35gm of Sotagliflozin. 5.7gm of Sotagliflozin (5.7gr, Sotagliflozin) and 28.5ml of Methyl ethyl ketone (28.5ml) were charged in round bottom flask, the slurry was stirred at 22-25°C for 5-10min gradually raised the temperature to 78°C then added 114 ml of n-Heptane (114ml) at 78°C over the period of 55min. Once the addition of n-heptane was completed, seeds of Form-2 (20 mg) were added, the slurry was gradually cooled down to 25-27°C over the period of 60 min. The obtained slurry was stirred for 2-3hrs at 25-27°C and the mass was kept overnight (without stirring) at 25-27°C then stirred for 3hr at 23 °C. The mass was filtered under vacuum and washed with 10ml of n-Heptane then kept for suction for 30min at 25-30°C. The material was dried at 50°C for 2hrs under vacuum to obtain Form-2 of Sotagliflozin. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping