Alternatived Products of [ 108413-55-4 ]
Product Details of [ 108413-55-4 ]
| CAS No. : | 108413-55-4 |
MDL No. : | MFCD01207052 |
| Formula : |
C9H8N2O2S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
208.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 108413-55-4 ]
Application In Synthesis of [ 108413-55-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 108413-55-4 ]
- 1
-
 [ 75-15-0 ]
[ 75-15-0 ]

-
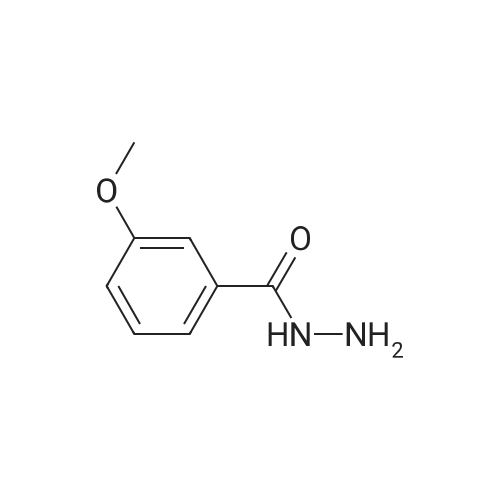 [ 5785-06-8 ]
[ 5785-06-8 ]

-
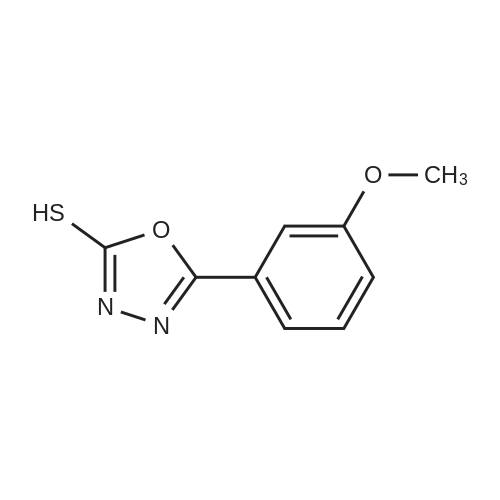 [ 108413-55-4 ]
[ 108413-55-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 87% |
With potassium hydroxide In ethanol for 38h; Reflux; |
|
| 86% |
With N,N-dimethyl-formamide for 18h; Sonication; Green chemistry; |
|
| 64% |
In N,N-dimethyl-formamide at 40 - 70℃; Inert atmosphere; |
4.10 4.1.2. General procedure for synthesis of 5-substituted 1,3,4-oxadiazole-2-thiols
General procedure: To a solution of acid hydrazide in anhydrous 5-15mL of DMF, carbon disulfide (2.5mL/mmol) was added at room temperature and under a nitrogen atmosphere. The reaction mixture was then heated to 40°C for 15min and then to 70°C for 4-8h until the reaction was completed. After completion, the reaction mixture was cooled to room temperature and poured dropwise into ice cold water. The solids formed were separated by filtration, washed with water and dried in vacuo. |
|
With potassium hydroxide In ethanol |
|
|
In ethanol for 5h; Reflux; |
|
|
Stage #1: carbon disulfide; 3-methoxybenzoic hydrazide With potassium hydroxide In ethanol Reflux;
Stage #2: With hydrogenchloride In ethanol; water |
|
|
With potassium hydroxide In ethanol Reflux; |
|
|
With potassium hydroxide In ethanol for 20h; Reflux; |
|
|
With triethylamine In methanol at 65 - 67℃; for 10h; |
|
|
Stage #1: carbon disulfide; 3-methoxybenzoic hydrazide With potassium hydroxide In ethanol
Stage #2: With hydrogenchloride In water |
|
|
With potassium hydroxide In ethanol; water at 95℃; for 16h; Inert atmosphere; |
|
|
Stage #1: 3-methoxybenzoic hydrazide With potassium hydroxide In ethanol for 0.166667h; Inert atmosphere;
Stage #2: carbon disulfide In ethanol Reflux;
Stage #3: In water at 70 - 80℃; |
4.1.2 General synthetic procedure for the key intermediate 5(a-c):
General procedure: The intermediate 5(a-c) were synthesized by two step reaction. Firstly, to a 100mL flask was added the intermediate compound 3(a-b) (1mmol) and milled potassium hydroxide (1.1mmol) in ethanol (5mL), stirred for 10min under nitrogen atmosphere. Later, slowly added carbon disulphide (2mmol) to the reaction mixture, solid will observed (if necessary, add ethanol) and heated to reflux for 4-5 h. The reaction progress was detected by using TLC technique. After completion of the reaction, the reaction mass was cooled to room temperature and concentrated under reduced pressure to afford a crude solid 4(a-d). The obtained crude solid was taken as such for further step. (0029) The obtained crude intermediate 4(a-d) was taken in water and heated to reflux at 70-80°C for 7-8 h. After completion of the reaction, the reaction mixture was gradually brought to room temperature followed cooled to 0 to 5°C. Then neutralised with 10% hydrochloric acid solution, the precipitated solid was filtered, and several times washed with cold water. The obtained solid was dried and used as it is for next step. |
|
With potassium hydroxide In ethanol for 12h; Reflux; |
|

Reference:
[1]Karabanovich, Galina; Zemanová, Júlia; Smutný, Tomáš; Székely, Rita; Šarkan, Michal; Centárová, Ivana; Vocat, Anthony; Pávková, Ivona; Čonka, Patrik; Němeček, Jan; Stolaříková, Jiřina; Vejsová, Marcela; Vávrová, Kateřina; Klimešová, Věra; Hrabálek, Alexandr; Pávek, Petr; Cole, Stewart T.; Mikušová, Katarína; Roh, Jaroslav
[Journal of Medicinal Chemistry, 2016, vol. 59, # 6, p. 2362 - 2380]
[2]Yarmohammadi, Elahe; Beyzaei, Hamid; Aryan, Reza; Moradi, Ashraf
[Molecular Diversity, 2021, vol. 25, # 4, p. 2367 - 2378]
[3]Kummari, Lalith K.; Butler, Mark S.; Furlong, Emily; Blundell, Ross; Nouwens, Amanda; Silva, Alberto B.; Kappler, Ulrike; Fraser, James A.; Kobe, Bostjan; Cooper, Matthew A.; Robertson, Avril A.B.
[Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 20, p. 5408 - 5419]
[4]Location in patent: scheme or table
Zareefa, Muhammad; Iqbal, Rashid; Zaidi, Javid Hussain; Arfan, Muhammad; Ali, Muhammad; Khan, Khalid M.
[Letters in Organic Chemistry, 2010, vol. 7, # 5, p. 411 - 414]
[5]Deora, Girdhar Singh; Karthikeyan, Chandrabose; Moorthy, N. S. Hari Narayana; Rathore, Vandana; Rawat, Arun K.; Tamrakar, Akhilesh K.; Srivastava; Trivedi, Piyush
[Medicinal Chemistry Research, 2013, vol. 22, # 11, p. 5344 - 5348]
[6]Li, Pei; Yin, Juan; Xu, Weiming; Wu, Jian; He, Ming; Hu, Deyu; Yang, Song; Song, Baoan
[Chemical Biology and Drug Design, 2013, vol. 82, # 5, p. 546 - 556]
[7]Ahmed, Muhammad Naeem; Yasin, Khawaja Ansar; Hameed, Shahid; Ayub, Khurshid; Haq, Ihsan-ul; Tahir, M. Nawaz; Mahmood, Tariq
[Journal of Molecular Structure, 2017, vol. 1129, p. 50 - 59]
[8]Wang, Zi-Zhen; Sun, Wen-Xue; Wang, Xue; Zhang, Ya-Han; Qiu, Han-Yue; Qi, Jin-Liang; Pang, Yan-Jun; Lu, Gui-Hua; Wang, Xiao-Ming; Yu, Fu-Gen; Yang, Yong-Hua
[Chemical Biology and Drug Design, 2017, vol. 90, # 2, p. 236 - 243]
[9]Ganesh Kumar; Gautham Shenoy; Kar, Sidhartha Sankar; Shenoy, Vishnu; Bairy, Indira
[Pharmaceutical Chemistry Journal, 2018, vol. 51, # 10, p. 907 - 917]
[10]Wang, Pei-Yi; Wang, Ming-Wei; Zeng, Dan; Xiang, Meng; Rao, Jia-Rui; Liu, Qing-Qing; Liu, Li-Wei; Wu, Zhi-Bing; Li, Zhong; Song, Bao-An; Yang, Song
[Journal of Agricultural and Food Chemistry, 2019, vol. 67, # 13, p. 3535 - 3545]
[11]Kahl, Dylan J.; Hutchings, Kim M.; Lisabeth, Erika Mathes; Haak, Andrew J.; Leipprandt, Jeffrey R.; Dexheimer, Thomas; Khanna, Dinesh; Tsou, Pei-Suen; Campbell, Phillip L.; Fox, David A.; Wen, Bo; Sun, Duxin; Bailie, Marc; Neubig, Richard R.; Larsen, Scott D.
[Journal of Medicinal Chemistry, 2019, vol. 62, # 9, p. 4350 - 4369]
[12]Vanjare, Balasaheb D.; Choi, Nam Gyu; Mahajan, Prasad G.; Raza, Hussain; Hassan, Mubashir; Han, Yohan; Yu, Seon-Mi; Kim, Song Ja; Seo, Sung-Yum; Lee, Ki Hwan
[Bioorganic and Medicinal Chemistry, 2021, vol. 41]
[13]Cheng, Wanqing; Fan, Jiangping; Guo, Yong; Han, Meiyue; Ma, Nannan; Yan, Xiaoting; Yang, Ruige
[Journal of Agricultural and Food Chemistry, 2021, vol. 69, # 51, p. 15544 - 15553]
- 2
-
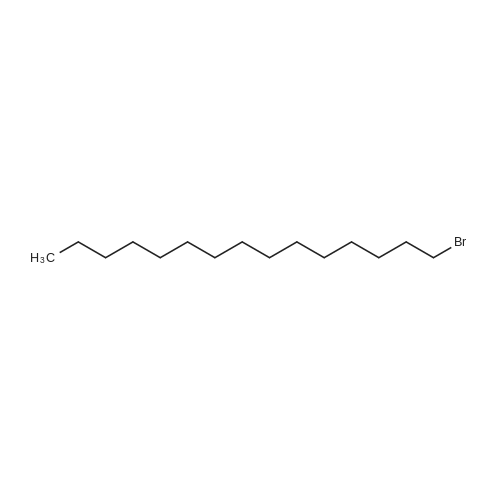 [ 629-72-1 ]
[ 629-72-1 ]

-
 [ 108413-55-4 ]
[ 108413-55-4 ]

-
5-(3-methoxyphenyl)-2-(pentadecylthio)-1,3,4-oxadiazole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 77% |
With potassium carbonate In acetone at 40℃; for 4h; |
2 2.2.1. General procedure for the synthesis of 1,3,4-oxadiazolederivatives (1-3)
General procedure: The synthesis of final products was accomplished trough following literature [31] protocol with slight modification. Alkyl bromide (2.5 mmol) was added to a suspension of 5-aryl-1,3,4-oxadiazole-2-thiol (1.5 mmol), potassium carbonate (2 mmol) and acetone (20 mL) in round bottom flask. The resultant mixture was stirred at 40 C for 4 h. After completion, the reaction mixture was diluted with water, extracted with DCM and finally washed with brine. Purification of products was carried out using flash column chromatography. |
Reference:
[1]Ahmed, Muhammad Naeem; Yasin, Khawaja Ansar; Hameed, Shahid; Ayub, Khurshid; Haq, Ihsan-ul; Tahir, M. Nawaz; Mahmood, Tariq
[Journal of Molecular Structure, 2017, vol. 1129, p. 50 - 59]
- 3
-
 [ 108413-55-4 ]
[ 108413-55-4 ]

-
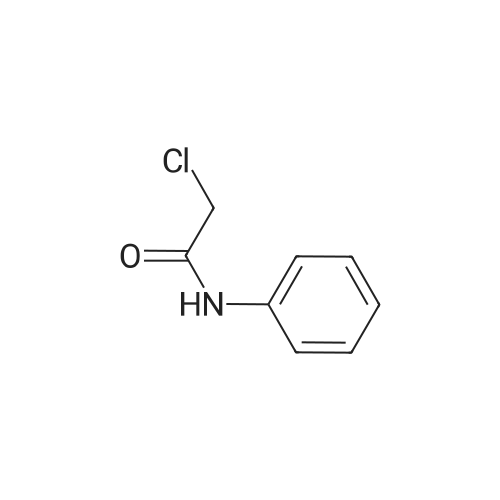 [ 587-65-5 ]
[ 587-65-5 ]

-
2-(5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-ylthio)-N-phenylacetamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 92% |
Stage #1: 5-(3-methoxyphenyl)-1,3,4-oxadiazole-2-thiol With potassium carbonate In N,N-dimethyl-formamide at 20℃; Inert atmosphere;
Stage #2: N-chloroacetyl-aniline In N,N-dimethyl-formamide at 20℃; Inert atmosphere; |
4.1.3 General procedure for the synthesis of the target compounds 9(a-l):
General procedure: As displayed in Scheme 1, an intermediate compound 5(a-l) (1mmol) and potassium carbonate (1.5mmol) in dimethylformamide (DMF) were stirred under nitrogen atmosphere at room temperature for 15-20min. Afterwards, added intermediate 8(a-d) (1mmol) to the above reaction mixture and stirred for 4-5 h. After completion of the reaction, ice cold water was added to the reaction mixture and stirred for 20min (solid was obtained). The obtained solid was filtered and several times washed with cold water and dried. The acquired crude solid was purified by column chromatography skill, when eluting with the hexane and ethyl acetate as a solvents to afford a pure 1,3,4-oxadiazole compounds 9(a-l) with an excellent yield. |
Reference:
[1]Vanjare, Balasaheb D.; Choi, Nam Gyu; Mahajan, Prasad G.; Raza, Hussain; Hassan, Mubashir; Han, Yohan; Yu, Seon-Mi; Kim, Song Ja; Seo, Sung-Yum; Lee, Ki Hwan
[Bioorganic and Medicinal Chemistry, 2021, vol. 41]
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









