Alternatived Products of [ 113283-94-6 ]
Product Details of [ 113283-94-6 ]
| CAS No. : | 113283-94-6 |
MDL No. : | MFCD11858354 |
| Formula : |
C12H18N2O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
222.28
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 113283-94-6 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 113283-94-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 113283-94-6 ]
- 1
-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
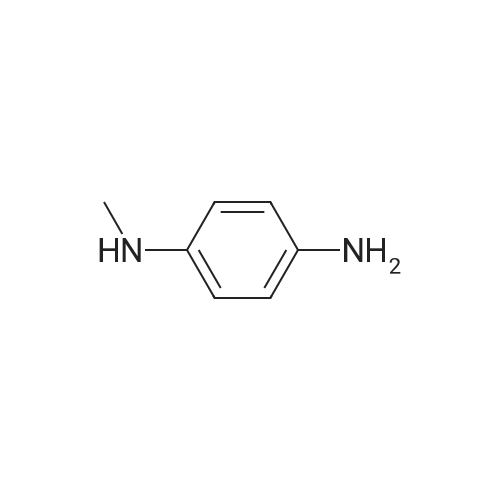 [ 623-09-6 ]
[ 623-09-6 ]

-
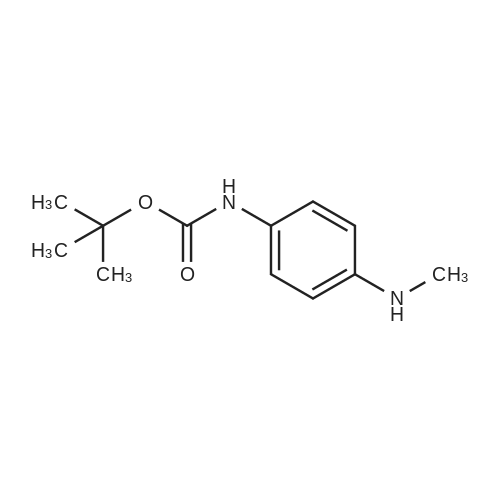 [ 113283-94-6 ]
[ 113283-94-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine at 20℃; for 3h; |
|
Reference:
[1]Hwang, Dong Jin; Yang, Jun; Xu, Huiping; Rakov, Igor M.; Mohler, Michael L.; Dalton, James T.; Miller, Duane D.
[Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 19, p. 6525 - 6538]
[2]Sundermann, Tom R.; Benzin, Clarissa V.; Dražić, Tonko; Klein, Christian D.
[European Journal of Medicinal Chemistry, 2019, vol. 176, p. 187 - 194]
- 2
-
 [ 623-09-6 ]
[ 623-09-6 ]

-
 [ 113283-94-6 ]
[ 113283-94-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: aq. Na2CO3
2: DMAP / acetonitrile / 15 h / Ambient temperature
3: 98 percent / H2 / Pd/C / methanol |
|
- 3
-
 [ 113283-94-6 ]
[ 113283-94-6 ]

-
 [ 62-50-0 ]
[ 62-50-0 ]

-
 [ 1111628-41-1 ]
[ 1111628-41-1 ]
- 4
-
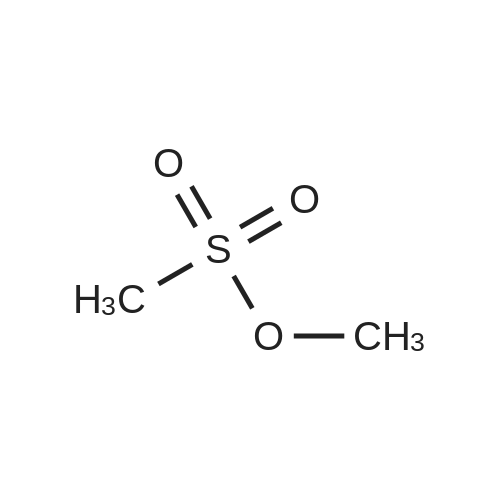 [ 66-27-3 ]
[ 66-27-3 ]

-
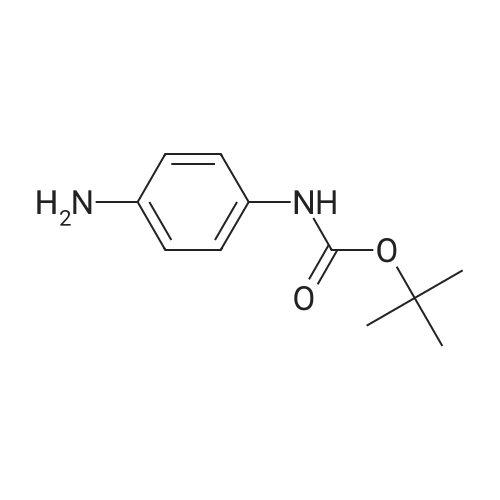 [ 71026-66-9 ]
[ 71026-66-9 ]

-
 [ 113283-94-6 ]
[ 113283-94-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine; In dichloromethane; at 0℃;Reflux; |
General Procedure for the Alkylation of Primary and Secondary Amines: Method C.The alkylating agent (10.0 mmol of <strong>[66-27-3]methyl methanesulfonate</strong> or ethyl methanesulfonate) and 10.0 mmol of TEA were added at 0 C. over a solution containing 10.0 mmol of the corresponding amine in DCM (12 mL). The resulting mixture was heated at reflux temperature for 15 h and after cooling it was diluted with 40 mL of DCM, washed with a 10% NaOH solution (2×15mL) and water (2×15 mL). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated under vacuum to give a residue that was purified by silica gel column chromatography, eluting with the appropriate hexane:EtOAc mixture. |
- 5
-
 [ 113283-94-6 ]
[ 113283-94-6 ]

-
 [ 623-09-6 ]
[ 623-09-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: N4-Boc-N1-methyl-1,4-phenylenediamine With trifluoroacetic acid In dichloromethane at 0℃; Reflux;
Stage #2: With sodium hydroxide In water |
General Procedure for the BOC-Deprotection and Preparation of the Starting Material Amines: Method D.A solution containing 10.0 mmol of the BOC-protected compound (11, 12 or 15) in 15 mL of TFA was stirred at room temperature for 2 h. Then, the solvent was eliminated under vacuum to generate the trifluoroacetate salt. This salt was redissolved in 20 mL of an aqueous solution of NaOH (2M) and washed with DCM (3×15 mL). The organic layer was washed with water (2×10 mL), dried over anhydrous Na2SO4, filtered and concentrated to give the corresponding free amine as an oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








