Alternatived Products of [ 1149388-20-4 ]
Product Details of [ 1149388-20-4 ]
| CAS No. : | 1149388-20-4 |
MDL No. : | MFCD16658635 |
| Formula : |
C10H11BrO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
259.10
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 1149388-20-4 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 1149388-20-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1149388-20-4 ]
- 1
-
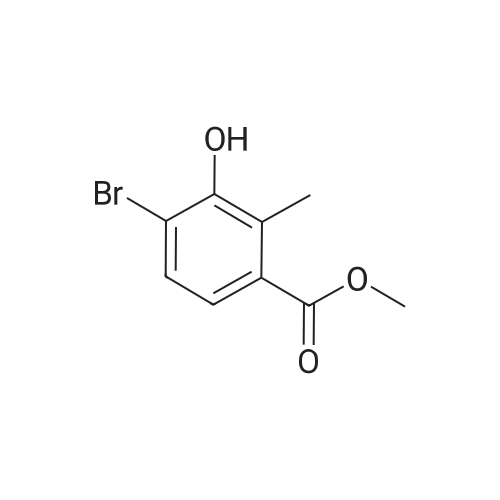 [ 1149388-19-1 ]
[ 1149388-19-1 ]

-
 [ 74-88-4 ]
[ 74-88-4 ]

-
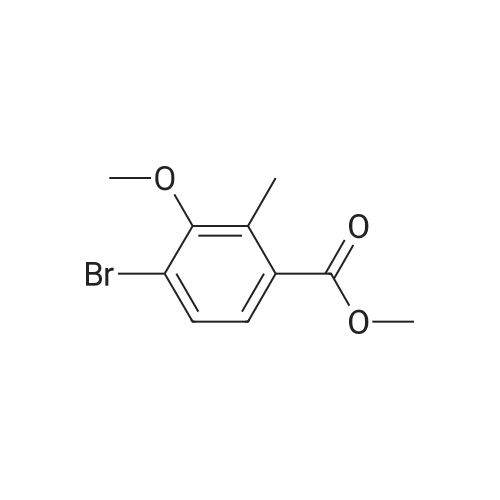 [ 1149388-20-4 ]
[ 1149388-20-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91% |
With potassium carbonate; In acetonitrile; at 50℃; for 5h; |
G1. 4-Bromo-3-methoxy-2-methyl-benzoic acid methyl ester A solution of <strong>[1149388-19-1]methyl 4-bromo-3-hydroxy-2-methylbenzoate</strong> (1.0 g, 4.08 mmoi) in dry MeCN (7 mL) was treated with potassium carbonate (0.677 g, 4.90 mmol), then iodomethane (0.762 mL, 12.24 mmoi) and the mixture heated at 50 C for 5 hrs. Solvents were removed in vacuo and the residue partitioned between EtOAc (20 mL) and water (20 mL). The aqueous layer was extracted with further EtOAc (2 x 20 mL) and the combined organics washed with brine (20 mL), dried (MgSO4), filtered and concentrated to give 4-bromo-3-methoxy-2-methyl-benzoic acid methyl ester (974 mg, 91 % yleld). [MH]+ = 259.0//261.0 |
| 81% |
With potassium carbonate; In N,N-dimethyl-formamide; acetone; at 20℃; |
<strong>[1149388-19-1]Methyl 4-bromo-3-hydroxy-2-methylbenzoate</strong> (1.51 g, 6.16 mmol), iodomethane (1.161 mL, 18.48 mmol) and potassium carbonate (2.55 g, 18.48 mmol) were dissolved in N,N-dimethylformamide (10 mL) and acetone (10 mL) and stirred at room temperature over night. Water was added and the aqueous phase was extracted with ethyl acetate and dichloromethane. The combined organic phases were washed with water, dried over magnesium sulfate and concentrated in vacuo to gave 1.3 g (81% yield) of the title compound.1H NMR (CDCl3) delta ppm 7.56-7.49 (m, 1H) 7.47-7.38 (m, 1H) 3.90 (s, 3H) 3.81 (s, 3H) 2.57 (s, 3H) |
| 81% |
With potassium carbonate; In N,N-dimethyl-formamide; acetone; at 20℃; |
<strong>[1149388-19-1]Methyl 4-bromo-3-hydroxy-2-methylbenzoate</strong> (1.51 g, 6.16 mmol), iodomethane (1.161 mL, 18.48 mmol) and potassium carbonate (2.55 g, 18.48 mmol) were dissolved in N,N- dimethylformamide (10 mL ) and acetone (10 mL) and stirred at room temperature over night. Water was added and the aqueous phase was extracted with ethyl acetate and dichloromethane. The combined organic phases were washed with water, dried over magnesium sulfate and concentrated in vacuo to gave 1.3 g (81% yield) of the title compound. 1H NMR (CDCl3) delta ppm 7.56 - 7.49 (m, 1 H) 7.47 - 7.38 (m, 1 H) 3.90 (s, 3 H) 3.81 (s, 3 H) 2.57 (s, 3 H) |
| 71% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; for 15h; |
STEP 3 : A solution of methyl 4-bromo-3 -hydroxy-2-methylbenzoate (610 mg, 2.5 mmol), cesium carbonate (1.22 g, 3.7 mmol) and iodomethane (162 mul, 2.6 mmol) in dimethylformamide (5 mL) was stirred at room temperature for 15 hours. The mixture was diluted with ethyl acetate, then washed with 5% aqueous lithium chloride then brine and dried over anhydrous sodium sulfate. Filtration and <n="404"/>concentration affored an orange residue, which was purified by silica gel column chromatography. Eluting with 15% diethyl ether in hexane, purified fractions were pooled and concentrated to afford 455 mg, 1.76 mmol (71%) of methyl 4-bromo-2- methyl-3-(methyloxy)benzoate as a colorless residue. 1H NMR (400 MHz, CDCl3): 7.52 (d, IH), 7.43 (d, IH), 3.88 (s, 3H), 3.80 (s, 3H), 2.55 (s, 3H). MS (EI) for Ci0HnBrO3: 260 (MH+). |

Reference:
[1]Patent: WO2016/83816,2016,A1 .Location in patent: Page/Page column 41-42
[2]Angewandte Chemie - International Edition,2019,vol. 58,p. 14625 - 14628
Angew. Chem.,2019,vol. 131,p. 14767 - 14770,4
[3]Patent: US2009/131468,2009,A1 .Location in patent: Page/Page column 41
[4]Patent: WO2009/64251,2009,A1 .Location in patent: Page/Page column 77
[5]Patent: WO2009/55077,2009,A1 .Location in patent: Page/Page column 401-402
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






