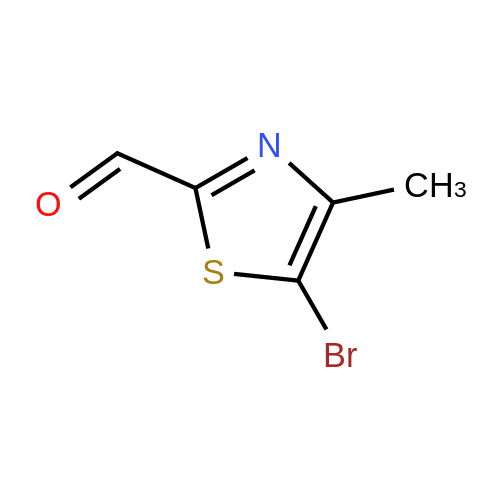
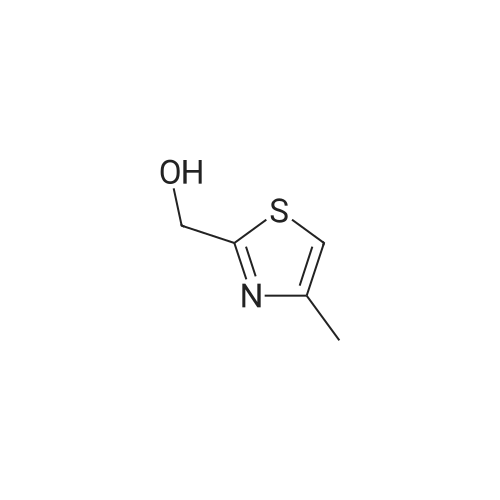
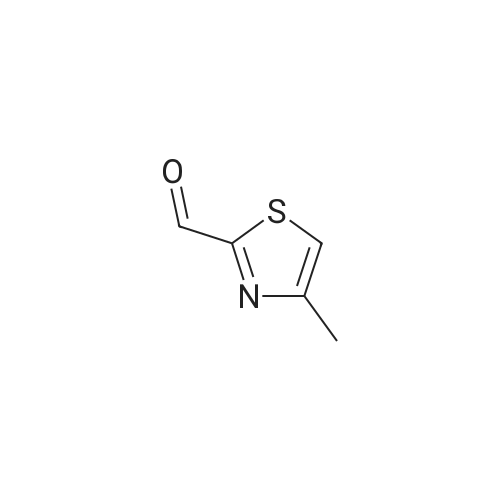
| 99% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 50℃; |
PREPARATION 115(5-Bromo-4-methyl-thiazol-2-yl)-methanolTo a solution of 4-methyl-thiazole (10 g, 100 mmol) in dry THF (300 mL) was added n- BuLi in hexane (60 mL, 150 mmol, 2.5 M) dropwise at -70C, and the mixture was stirred at -70C for 1.5 hour, DMF (12 mL) was added to the slurry over 15 min, and the mixture was stirred at -70C for 3 hours. TLC (petroleum etherEtOAc 10:1 ) indicated the reaction was completed. The mixture was warmed to 0C, and poured onto wet-ice. The mixture was acidified by 2N HCI to pH ~ 4, and extracted with EtOAc (100 mL chi 3). The combined organic layers were washed with brine (100 mL), dried over sodium sulfate, and concentrated in vacuum to give 4-methyl-thiazole-2-carbaldhyde (10 g, 78.7%) as brown oil.To a solution of 4-methyl-thiazole-2-carbaldhyde (10.0 g, 78 mmol) in dry THF (80 mL) was added NaBH4 (1.49 g, 39 mmol), and the mixture was stirred at room temperature for 2 hours. TLC (petroleum etherEtOAc 2:1 ) indicated the reaction was completed. The mixture was diluted with NH4CI solution (50 mL) and the mixture was filtered. The filtrate was extracted with EtOAc (50 mL chi 3). The combined organic layers were washed with brine (50 mL), dried over sodium sulfate, and concentrated in vacuum to give the residue which was purified by a silica gel column eluting with petroleum etherEtOAc 3:1 to give (4-Methyl-thiazol-2-yl)-methanol (7 g, 70%) as a yellow oil.To a solution of <strong>[13750-63-5](4-methyl-thiazol-2-yl)-methanol</strong> (7.0 g, 54.3 mmol) in DMF (80 mL) was added NBS (10.6 mg, 59.6 mmol), and the mixture was stirred at 50C overnight. TLC (petroleum etherEtOAc 2:1 ) indicated the reaction was completed. The mixture was diluted with H20 (50 mL) and extracted with EtOAc (50 mL chi 3). The combined organic layers were washed with brine (50 mL 3), dried over sodium sulfate, and concentrated in vacuum to give the residue which was purified by a silica gel column (petroleum etherEtOAc 10:1 ) to give the title compound (1 1.2 g, 99%) as a brown solid. |
| 78% |
With N-Bromosuccinimide; In acetonitrile; at 20℃; for 2h; |
To a solution of 29 (10.0 g, 77 mmol) in MeCN (387 mL) was added NBS (13.8 g, 77.0 mmol) at room temperature. After being stirred for 2 h at room temperature, the reaction mixture was concentrated in vacuo. The residue was diluted with EtOAc, washed with saturated aqueous NH4Cl solution, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (silica gel, eluted with 33% EtOAc in hexane) to give 12.6 g (78%) of 30 as yellow solid. 1H NMR (400 MHz, CDCl3)d 2.37 (3H, s), 2.74 (1H, brs), 4.85 (2H, s). |
| 75% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 50℃; for 2h; |
To a solution of thiazole 21a (720 mg, 5.58 mmol) in DMF (15 mL) was added NBS (1.1 g, 6.14 mmol) and the mixture was stirred at 50C for 2 hours. After the completion of the reaction, the mixture was diluted with water and was extracted with ethyl acetate. The combined organic extracts were washed with brine, dried over Na2SO4 and concentrated under reduced pressure to give a residue which was purified in ISCO max gradient 40% EtOAc/hexane to give the pure product (870 mg, 75% yield). 1H NMR (CDCl3, 400 MHz) 8: 4.83 (s, 2H), 4.01 (bs, 1H), 2.36 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping