|
With pyridine; at 20℃; for 1.0h;Product distribution / selectivity; |
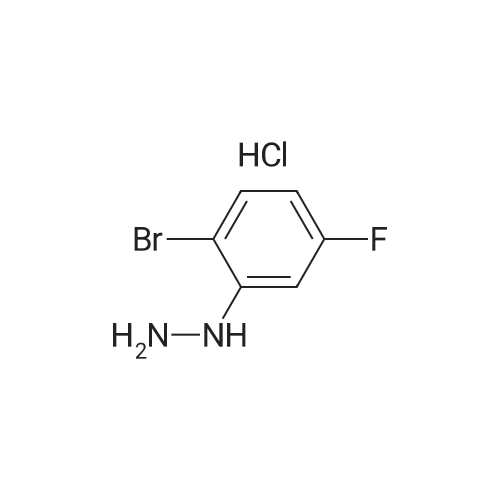
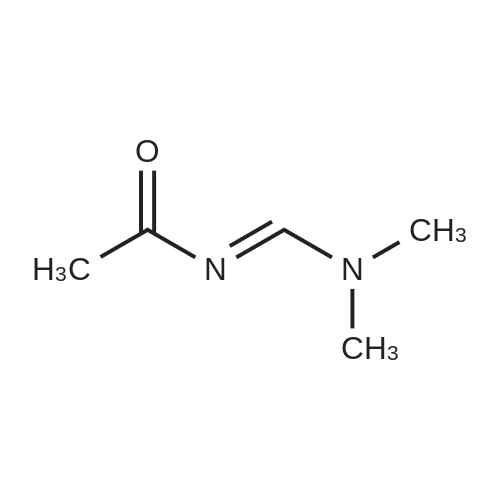
To a solution of <strong>[60481-35-8](2-bromo-5-fluoro-phenyl)-hydrazine hydrochloride</strong> (1.21 g, 5 mmol) in pyridine (3 mL) was added a solution of N-((dimethylamino)methylene)acetamide (600 mg, 5.26 mmol) in pyridine (2 mL) and the mixture stirred at room temperature under nitrogen for 1 h. The precipitate formed was collected, washed with CH2Cl2 and then with ether to obtain 1.15 g (3.16 mmol, 63% yield) of the title compound which was contaminated with 1 mole of dimethylamine hydrochloride as a white crystalline powder: HPLC: 2.22 min (AP 84% at 220 nm); LC/MS m/z 274/276 (M+H); 1H NMR (DMSO-d6, 500 MHz) delta ppm 2.00 (3H, s, 9-Me), 2.51 (6H, s, 2 N-CH3), 6.45 (1H, dt, J=8.5, 3 Hz, 5-CH), 6.89 (1H, dd, J=12, 3 Hz, 3-CH), 7.43 (1H, dd, J=8.5, 6 Hz, 6-CH), 8.68 (1H, d, J=9.5 Hz, 7-CH), 8.88 (2H, br.s, NH2+), 9.24 (1H, s, 7-NH), 10.54 (1H, d, J=9.5 Hz, 2-NH); No signals from the rotational isomer were observed; 13C NMR (CDCl3, 125.8 Hz) deltappm 22.6 (9-CH3), 33.9 (2 NCH3), 99.6 (d, J=28 Hz, 3-CH), 99.7 (1-C), 105.0 (d, J=24 Hz, 5-CH), 133.5 (d, J=10.6 Hz, 6-CH), 137.1 (7-CH), 144.9 (d, J=11.6 Hz, 2-C), 162.4 (d, J=244 Hz, 4-CF), 168.5 (8-CO). No signals from the rotational isomer were observed; Anal. calcd for C9H9BrFN3O.Me2NH.HCl.1/2H2O: C36.24, H4.99, N15.37, found C35.88, H4.87, N15.23. An analytical sample of the title compound without contamination of dimethylamine hydrochloride was obtained by column purification (SiO2, 10-15% EtOAc-CH2Cl2): TLC Rf 0.55 (20% EtOAc-CH2Cl2); HPLC: 2.17 min (AP 88% at 220 nm); LC/MS m/z 274/276 (M+H); 1H NMR (DMSO-d6, 500 MHz) delta ppm 1.99 (3H, s, 9-Me), 6.45 (1H, dt, J=8.5, 3 Hz, 5-CH), 6.88 (1H, dd, J=12, 3 Hz, 3-CH), 7.43 (1H, dd, J=9, 6 Hz, 6-CH), 8.69 (1H, d, J=9.5 Hz, 7-CH), 9.23 (1H, s, 7-NH), 10.52 (1H, d, J=9.5 Hz, 2-NH); About 16% of rotational isomer was also observed as a set of minor peaks: 1H NMR (DMSO-d6, 500 MHz) delta ppm 2.11 (3H, s, 9'-Me), 6.58 (1H, dt, J=8.5, 3 Hz, 5'-CH), 7.10 (1H, dd, J=1 1.6, 3 Hz, 3'-CH), 7.29 (1H, d, J=2.4 Hz, 7'-CH), 7.50 (1H, dd, J=8.7, 6 Hz, 6'-CH), 8.35 (1H, s, 2'-NH), 10.70 (1H, s, 7'-NH); 13C NMR (DMSO-d6, 125.8 Hz) deltappm 22.6 (9-CH3), 99.7 (d, J=29 Hz, 3-CH), 99.8 (d, J=3 Hz, 1-C), 105.0 (d, J=24 Hz, 5-CH), 133.6 (d, J=10.6 Hz, 6-CH), 137.1 (7-CH), 144.9 (d, J=11.6 Hz, 2-C), 162.5 (d, J=241 Hz, 4-CF), 168.5 (8-CO); 13C NMR (DMSO-d6, 125.8 Hz) delta ppm 22.9 (9'-CH3), 100.7 (d, J=3 Hz, 1'-C), 101.6 (d, J=29 Hz, 3'-CH), 106.8 (d, J=24 Hz, 5'-CH), 133.6 (d, J=9.6 Hz, 6'-CH), 162.4 (d, J=242 Hz, 4'-CF), 168.6 (8'-CO); HRMS (ESI) calcd for C9H10BrFN3O (M+H) 273.9991, found 274.0004 (delta+4.6 ppm); Anal. calcd for C9H9BrFN3O: C39.43, H3.31, N29.15, found C39.67, H2.99, N29.09. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping