| 93% |
With potassium acetate In N,N-dimethyl acetamide at 110℃; for 7h; Inert atmosphere; |
12.2
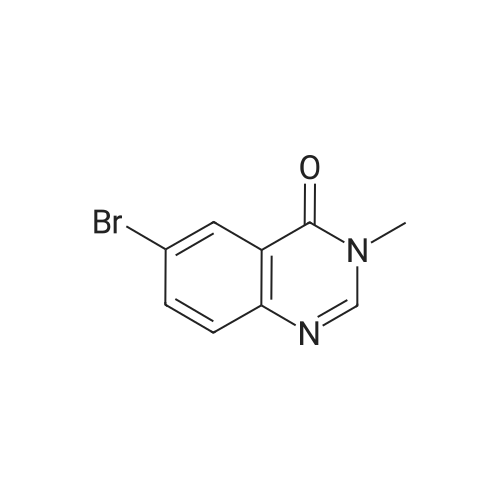
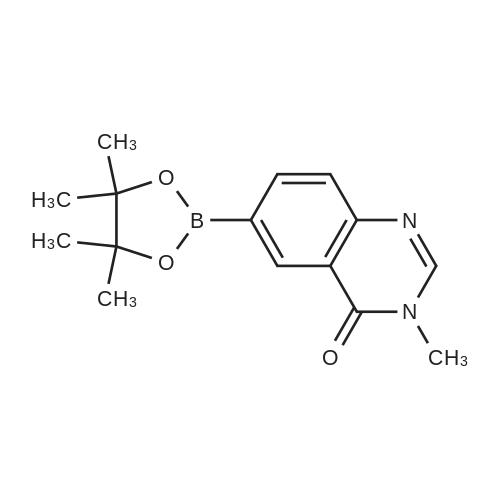
Step 2: 3-methyl-6-(4.4.5.5-tetramethyl-1.3.2-dioxaborolan-2-yl)αuinazolin-4(3H)-one; A mixture of 6-bromo-3-methylquinazolin-4(3H)-one (2 g, 8.3 mmol), bis(pinacolato)diborane (2.43 g, 9.57 mmol), dichloro[1,1 ,'-bis(diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (0.35 g, 0.11 mmol), 1 ,1 ,'- bis(diphenylphosphino)ferrocene (0.09 g, 0.16 mmol), potassium acetate (2.5 g, 25.5 mmol) and N.N-dimethylsulfoxide (30 ml_) was heated at 110 0C for 7 h under nitrogen atmosphere. The mixture was cooled to room temperature and diluted with ethyl acetate. Water (15 ml_) was added and the layers were separated. The organic layer was washed 3 times with water (15 ml_), washed with brine and dried over sodium sulfate. The organic layer was and concentrated to a black solid. The solid was stirred in a hexane/ diethyl ether mixture to give the title compound (2.2g, 93%) as fine grey powder. 1H NMR (400 MHz, CHLOROFORM-d) δ ppm: 8.77 (d, 1 H, J=1.0 Hz), 8.11 (dd, 1 H, J=8.2, 1.6 Hz), 8.04 (s, 1 H), 7.64 (dd, 1 H, J=8.2, 0.4 Hz), 3.57 (s, 3H), 1.34 (s, 12H). |
| 62% |
With [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II); potassium acetate In 1,4-dioxane at 1120℃; for 2h; Inert atmosphere; |
20
A solution of methylquinazolinone lv (2 g, 8.4 mmol) in l,4-dioxane (60 mL) is degassed under nitrogen for 10 minutes. Potassium acetate (2.8 g, 28.6 mmol), (0268) bis(pinacolato)diboron (2.5 g, 9.9 mmol), and [1,1’- bis(diphenylphosphino)ferrocene]dichloropalladium(II) (Pd(dppf)Cl2) (600 mg, 0.82 mmol) are added. The reaction is heated to 120 °C for 12 hours, then cooled to room temperature, and the solid removed by filtration. The filtrate is concentrated, and the residue is purified by flash column chromatography (10% methanol in DCM) to give dioxaborolane xxxiii (1.5 g, 5.2 mmol) in 62% yield. |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate In 1,4-dioxane at 100℃; for 1h; Sonication; Cooling with ice; |
VI Synthesis of 3-Methyl-6-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)quinazolin-4(3//)- one (Precursor IX).
To the 500 mL round-bottom flask were added 6-bromo-3-methylquinazolin-4(3H)- one (10 grams, 41 mmol), bis(pinacolato)diboron (12.7 grams, 50 mmol), Pd(dppf)Cl2*DCM (1.7 grams, 2 mmol), potassium acetate (12.3 grams, 12 mmol) and l,4-dioxane (236 mL). After being briefly stirred for several minutes, the reaction mixture was degassed using sonicator, subject to vacuum and then purged with nitrogen. This vacuum/nitrogen cycle was performed for three times. The reaction mixture was then heated at l00°C. Reaction is complete after 1 hour and no starting material remained according to LCMS. The reaction mixture was filtered through sand using filter paper, concentrated and purified on silica using ethyl acetate and hexanes. The product was obtained in 85% yield. NMR (400 MHz, Chloroform-d): d 8.78 (d, J = 1.5 Hz, 1H), 8.12 (dd, J = 8.1, 1.5 Hz, 1H), 8.07 (s, 1H), 7.66 (d, J = 8.1 Hz, 1H), 3.59 (s, 3H), 1.36 (s, 12H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping