| 60% |
With copper(l) iodide; potassium carbonate; In tetrahydrofuran; N,N-dimethyl-formamide; at 70 - 120℃;Inert atmosphere of nitrogen;Product distribution / selectivity; |
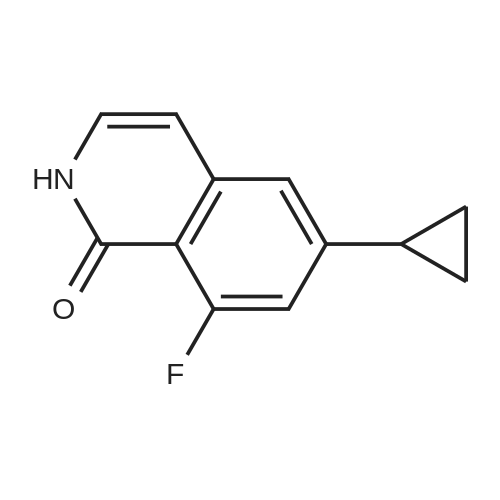
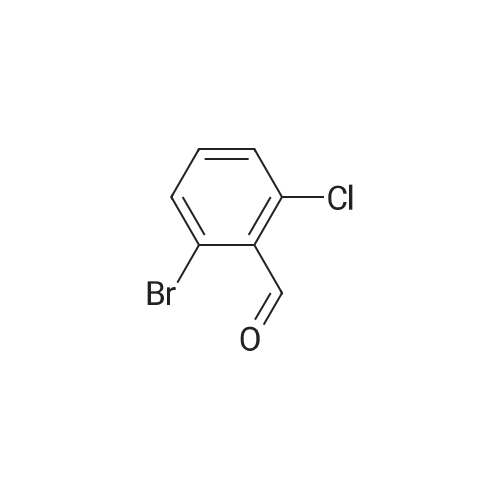
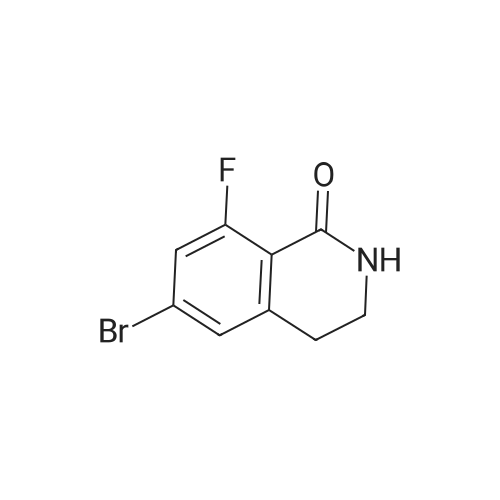
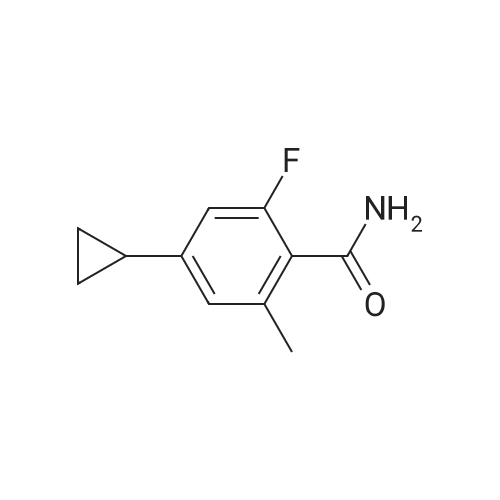
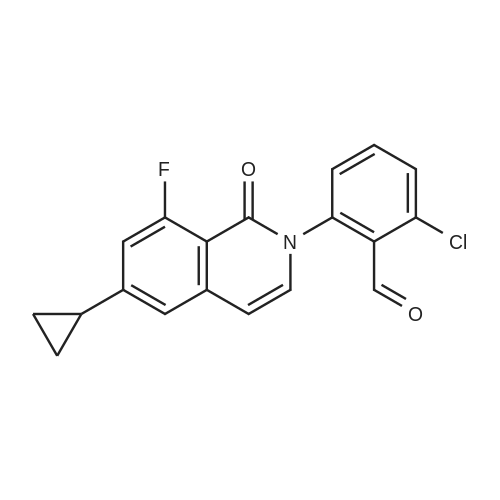
In a 1 L reactor, 6-cyclopropyl-8-fluoroisoquinolin-1(2H)-one (65 g, 0.32 mol), <strong>[64622-16-8]2-bromo-6-chlorobenzaldehyde</strong> (84.2 g, 0.38 mol), copper(I) iodide (12.2 g, 64.0 mmol) and potassium carbonate (88.4 g, 0.64 mol) were charged. The reactor was evacuated and backfilled with Nitrogen. This sequence was repeated three times. Then, DMF (650 ml) was added and the resulting mixture was heated to 120 C. for 20 hr. The reaction mixture was cooled down to about 70 C., and THF (975 ml) was added. Then, the resulting mixture was allowed to cool down to ambient temperature, followed by filtration through Celite pad. The filtrate was concentrated down under vacuum with distilling THF off. Crystallization was performed with DMF/IPA/H2O (10/5/2) at around 60 C., and the material was aged overnight with slow cooling. The desired product was collected by filtration and washed with IPA/H2O. The filter cake was dried under vacuum at 70 C. overnight to afford 65.4 g of the title compound (60% isolated yield) as a yellow solid. MS (ESI) 341, 343 (M+H)-. |
| 2.7 g |
With copper(l) iodide; potassium carbonate; In N,N-dimethyl-formamide; at 110℃; for 24h;Inert atmosphere; |
Under nitrogen atmosphere, <strong>[64622-16-8]2-bromo-6-chlorobenzaldehyde</strong> (3.65 g, 16.63 mmol), potassium carbonate (3.54 g, 25.6 mmol) and copper(I) iodide (0.49 g, 2.56 mmol) were added to a solution of 6-cyclopropyl-8-fluoroisoquinolin-1(2H)-one (2.6 g, 12.8 mmol) in DMF (25 mL), and stirred at 110 C. for 1 day. The reaction mixture was diluted with ethyl acetate (200 mL), filtered to remove insoluble material, and then the filtrate was washed with water and brine, dried over sodium sulfate, filtered and concentrated. The crude material was purified by chromatography on silica gel, eluted with hexane/ethyl acetate to afford 2-chloro-6-(6-cyclopropyl-8-fluoro-1-oxoisoquinolin-2(1H)-yl)benzaldehyde (2.7 g). 1H NMR (400 MHz, DMSO-d6) delta10.18 (s, 1H), 7.84-7.78 (m, 1H), 7.75 (dd, J=8.2, 1.3 Hz, 1H), 7.49 (dd, J=7.8, 1.2 Hz, 1H), 7.41 (d, J=7.5 Hz, 1H), 7.27 (d, J=1.6 Hz, 1H), 7.00 (dd, J=13.3, 1.6 Hz, 1H), 6.64 (dd, J=7.5, 2.2 Hz, 1H), 2.14-2.01 (m, 1H), 1.14-1.06 (m, 2H), 0.92-0.83 (m, 2H); LCMS (m/z): 342.1 [M+H]+. |
| 2.7 g |
With copper(l) iodide; potassium carbonate; In N,N-dimethyl-formamide; at 110℃; for 24h;Inert atmosphere; |
Under nitrogen atmosphere, <strong>[64622-16-8]2-bromo-6-chlorobenzaldehyde</strong> (3.65 g, 16.63 mmol), potassium carbonate (3.54 g, 25.6 mmol) and copper(I) iodide (0.49 g, 2.56 mmol) were added to a solution of 6-cyclopropyl-8-fluoroisoquinolin-1(2H)-one (2.6 g, 12.8 mmol) in DMF (25 mL), and stirred at 110 C. for 1 day. The reaction mixture was diluted with ethyl acetate (200 mL), filtered to remove insoluble material, and then the filtrate was washed with water and brine, dried over sodium sulfate, filtered and concentrated. The crude material was purified by chromatography on silica gel, eluted with hexane/ethyl acetate to afford 2-chloro-6-(6-cyclopropyl-8-fluoro-1-oxoisoquinolin-2(1H)-yl)benzaldehyde (2.7 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping