Alternatived Products of [ 1255147-00-2 ]
Product Details of [ 1255147-00-2 ]
| CAS No. : | 1255147-00-2 |
MDL No. : | MFCD18064657 |
| Formula : |
C14H16O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
216.28
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 1255147-00-2 ]
Application In Synthesis of [ 1255147-00-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1255147-00-2 ]
- 1
-
 [ 94723-89-4 ]
[ 94723-89-4 ]

-
 [ 105-53-3 ]
[ 105-53-3 ]

-
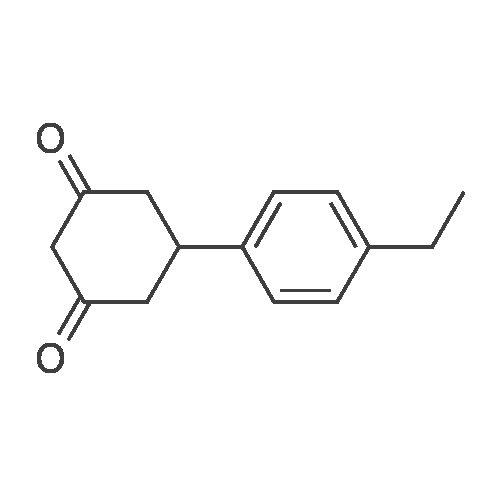 [ 1255147-00-2 ]
[ 1255147-00-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: 4-ethylbenzalacetone; diethyl malonate With sodium ethanolate In ethanol for 12h;
Stage #2: With sodium hydroxide In ethanol; water for 12h;
Stage #3: With hydrogenchloride In diethyl ether; water |
4.2. General procedure to prepare cyclohexane 1,3-dione (CHD) analogs7
General procedure: All aldehydes were previously prepared or purchased. Aldehyde 1 (5.0 mmol) was dissolved in THF (20 mL), and 1-(triphenylphosphoranylidene)-2-propanone (1.60 g, 5.0 mmol) was added. The mixture was allowed to stir at room temperature for 12 h. Solvent was removed under vacuum, and the residue was purified by flash column chromatography to provide 2, which was used directly in the next step, in almost quantitative yield.Compound 2 (2.0 mmol) and diethyl malonate (2.0 mmol) were dissolved in EtOH (5 mL), and a NaOEt solution (21 wt % in ethanol; 1.50 mL, 4.0 mmol) was added. After being stirred for 12 h, 3 N aq NaOH (20 mL) was added, and stirring was continued for another 12 h. Aqueous 3 N HCl was added until the solution turned cloudy. Ether was used to extract the cloudy mixture until the aqueous layer was clear. The combined organic portions were combined and allowed to stir for 4 h until decarboxylation was complete. Heating might be required in some cases when decarboxylation was not complete at room temperature. The solvent was evaporated, and the crude product was then purified by recrystallization or flash chromatography. The CHD product was typically a white solid, obtained in about an 80% yield, and had an Rf = 0.6 (dichloromethane/methanol = 8:1). |
Reference:
[1]Location in patent: experimental part
Zhang, Wei; Benmohamed, Radhia; Arvanites, Anthony C.; Morimoto, Richard I.; Ferrante, Robert J.; Kirsch, Donald R.; Silverman, Richard B.
[Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 2, p. 1029 - 1045]
- 2
-
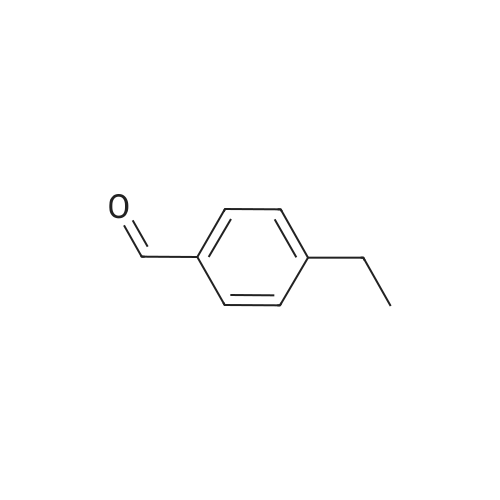 [ 4748-78-1 ]
[ 4748-78-1 ]

-
 [ 1255147-00-2 ]
[ 1255147-00-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: tetrahydrofuran / 12 h / 20 °C
2.1: sodium ethanolate / ethanol / 12 h
2.2: 12 h |
|
|
Multi-step reaction with 2 steps
1.1: sodium hydroxide / water / 20 °C
2.1: sodium; ethanol / 54 h / 20 °C / Reflux
2.2: 6 h / Reflux
2.3: 3 h / Reflux |
|
Reference:
[1]Zhang, Wei; Benmohamed, Radhia; Arvanites, Anthony C.; Morimoto, Richard I.; Ferrante, Robert J.; Kirsch, Donald R.; Silverman, Richard B.
[Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 2, p. 1029 - 1045]
[2]Current Patent Assignee: FOND JEROME LEJEUNE - WO2013/68592, 2013, A1
- 3
-
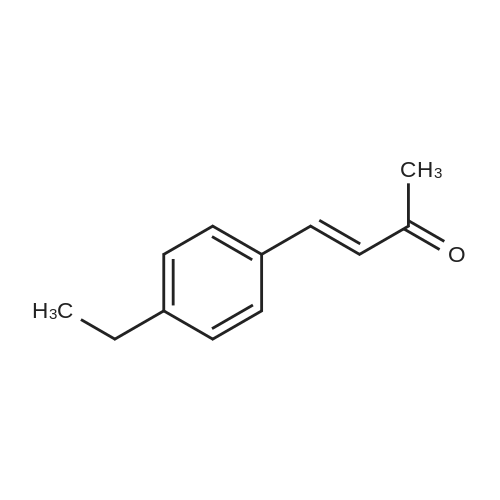 [ 114498-65-6 ]
[ 114498-65-6 ]

-
 [ 105-53-3 ]
[ 105-53-3 ]

-
 [ 1255147-00-2 ]
[ 1255147-00-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 6% |
Stage #1: (E)-4-(4-ethylphenyl)-but-3-en-2-one; diethyl malonate With ethanol; sodium at 20℃; for 54h; Reflux;
Stage #2: With sodium hydroxide In water for 6h; Reflux;
Stage #3: With sulfuric acid In water for 3h; Reflux; |
6.2 Step 2 Compound 13 : 5-(4-Ethyl-phenyl)-cyclohexane-1,3-dione
MW : 216.28 Formula : CI 4H1602 LCMS : 96.9% MH+=217 1.9mL (12.51mmol) of diethylmalonate and 2.18g (12.51mmol) of Compound 12 were added to a solution of 288mg (12.52mmol) of sodium in 40mL of EtOH. It was refluxed for 6h and then stirred 48h at room temperature. The reaction mixture was concentrated, 20mL of NaOH 2N were added and it was refluxed for 6 more hours. It was then acidified with H2S04 6N and then brought to reflux for 3h. The reaction mixture was extracted with AcOEt, the combined organic layers were dried over MgS04 and concentrated under vacuum. The residue was purified by silica gel flash chromatography (DCM/MeOH 99/1) to afford 173mg (6%) of Compound 13 as a white powder. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







