| 71% |
With acetic acid; at 20℃; |
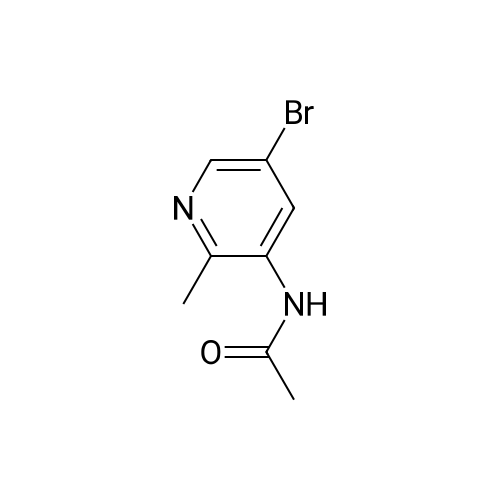
[0201] Step 2. N-(5-Bromo-2-methyl-pyridin-3-yl)-acetamide. A solution of 5-bromo-2- methyl-pyridin-3-ylamine (3 g, 16.04 mmol, 1.00 equiv) in acetic anhydride (20 mL) and acetic acid (10 mL) was stirred overnight at rt. The resulting mixture was concentrated under vacuum to give 2.6 g (7 1%) of the title compound as a light yellow solid. LC/MS (Method I, ESI): RT= 1.05 mm, m/z = 229.0; 231.0 [M+Hf?. |
| 71% |
In acetic acid; at 20℃; |
[0209] Step 2. N-(5-Bromo-2-methyl-pyridin-3-yl)-acetamide. A solution of 5-bromo-2- methyl-pyridin-3-ylamine (3 g, 16.04 mmol, 1.00 equiv) in acetic anhydride (20 mL) and acetic acid (10 mL) was stirred overnight at rt. The resulting mixture was concentrated under vacuum to give 2.6 g (71 ) of the title compound as a light yellow solid. LC/MS (Method I, ESI): RT= 1.05 min, m/z = 229.0; 231.0 [M+H]+. |
| 63% |
With triethylamine; In dichloromethane; at 0 - 20℃; |
To a solution of 5-bromo-2-methylpyridin-3-amine (10.7 g, 57.5 mmol) in dichloromethane (575 mL) was added acetic anhydride (12 mL, 126.5 mmol) at 0 C. followed by triethylamine (22 mL, 158 mmol). The mixture was allowed to warm to ambient temperature and stirred for 18 hours at which point a further equivalent of acetic anhydride (6 mL, 63 mmol) was added. The mixture was stirred at ambient temperature for a further 72 hours. The reaction mixture was quenched with a saturated aqueous solution of sodium bicarbonate (500 mL) and the organic phase washed with saturated aqueous sodium chloride (500 mL), dried over magnesium sulfate and concentrated in vacuo to give a brown solid. This solid was triturated with 30% ethyl acetate in hexanes to yield N-(5-bromo-2-methylpyridin-3-yl)acetamide as an off-white solid, (8.28 g, 36 mmol, 63%). 1HNMR (400 MHz, CD3OD): delta ppm 8.31 (s, 1H), 8.18 (s, 1H), 2.43 (s, 3H), 2.18 (s, 3H). |
| 63% |
With triethylamine; In dichloromethane; at 20℃; for 90h; |
To a solution of 5-bromo-2-methylpyridin-3-amine (10.7 g, 57.5 mmol) in dichloromethane (575 mL) was added acetic anhydride (12 mL, 126.5 mmol) at 0 C followed by triethylamine (22 mL, 158 mmol). The mixture was allowed to warm to ambient temperature and stirred for 18 hours at which point a further equivalent of acetic anhydride (6 mL, 63 mmol) was added. The mixture was stirred at ambient temperature for a further 72 hours. The reaction mixture was quenched with a saturated aqueous solution of sodium bicarbonate (500 mL) and the organic phase washed with saturated aqueous sodium chloride (500 mL), dried over magnesium sulfate and concentrated in vacuo to give a brown solid. This solid was triturated with 30 % ethyl acetate in hexanes to yield N-(5-bromo-2-methylpyridin-3-yl)acetamide as an off-white solid, (8.28 g, 36 mmol, 63 %). 1HNMR (400 MHz, CD3OD): ppm 8.31 (s, 1 H), 8.18 (s, 1 H), 2.43 (s, 3H), 2.18 (s, 3H). |
| 63% |
With triethylamine; In dichloromethane; at 0 - 20℃; |
To a solution of 5-bromo-2-methylpyridin-3-amine (10.7 g, 57.5 mmol) in dichloromethane (575 mL) was added acetic anhydride (12 mL, 126.5 mmol) at 0 C. followed by triethylamine (22 mL, 158 mmol). The mixture was allowed to warm to ambient temperature and stirred for 18 hours at which point a further equivalent of acetic anhydride (6 mL, 63 mmol) was added. The mixture was stirred at ambient temperature for a further 72 hours. The reaction mixture was quenched with a saturated aqueous solution of sodium bicarbonate (500 mL) and the organic phase washed with saturated aqueous sodium chloride (500 mL), dried over magnesium sulfate and concentrated in vacuo to give a brown solid. This solid was triturated with 30% ethyl acetate in hexanes to yield N-(5-bromo-2-methylpyridin-3-yl)acetamide as an off-white solid (8.28 g, 63%). 1HNMR (400 MHz, CD3OD, delta): 8.31 (s, 1H), 8.18 (s, 1H), 2.43 (s, 3H), 2.18 (s, 3H). |
| 63% |
With triethylamine; In dichloromethane; at 0 - 20℃; |
To a solution of 5-bromo-2-methylpyridin-3-amine (M-2) (10.7 g, 57.5 mmol) in dichloromethane (575 mL) was added acetic anhydride (12 mL, 126.5 mmol) at 0 C, followed by triethylamine (22 mL, 158 mmol). The mixture was allowed to warm to ambient temperature and stirred for 18 hours at which point a further equivalent of acetic anhydride (6 mL, 63 mmol) was added. The mixture was stirred at ambient temperature for a further 18 hours. The reaction mixture was quenched with a saturated aqueous solution of sodium bicarbonate (500 mL) and the organic phase washed with saturated aqueous sodium chloride (500 mL), dried over magnesium sulfate and concentrated in vacuo to give a brown solid. This solid was triturated with 30% ethyl acetate in hexanes to yield the title compound as an off-white solid, (8.28 g, 36 mmol, 63%). 1HNMR (400 MHz, CD3OD): delta ppm 8.31 (s, 1H), 8.18 (s, 1H), 2.43 (s, 3H), 2.18 (s, 3H). LCMS (ESI) calc'd for C8H9BrN20 [M+H]+: 228.99, found: 229, 230. |
| 63% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 36h; |
To a solutionof 5-bromo-2-methylpyridin-3-amine (M-2) (10.7 g, 57.5 mmol) in dichioromethane (575mL) was added acetic anhydride (12 mL, 126.5 mmol) at 0 C, followed by triethylamine (22mL, 158 mmol). The mixture was allowed to warm to ambient temperature and stirred for 18hours at which point a further equivalent of acetic anhydride (6 mL, 63 mmol) was added.The mixture was stirred at ambient temperature for a further 18 hours. The reaction mixture was quenched with a saturated aqueous solution of sodium bicarbonate (500 mL) and the organic phase washed with saturated aqueous sodium chloride (500 mL), dried over magnesium sulfate and concentrated in vacuo to give a brown solid. This solid was trituratedwith 30% ethyl acetate in hexanes to yield the title compound as an off-white solid, (8.28 g,36 mmol, 63%). ?HNMR (400 MHz, CD3OD): oe ppm 8.31 (s, 1H), 8.18 (s, 1H), 2.43 (s, 3H),2.18 (s, 3H). LCMS (ESI) calc?d for C8H9BrN2O [M+H]: 228.99, found: 229, 230. |
|
|
To a solution of T-4 (12.1 g, 64.6 mmol) in DCM (50 mL) is added acetic anhydride (9.50 mL, 98.5 mmol) followed by Et3N (20.0 mL, 143.7 mmol). After 18 hours, the reaction is concentrated and the residue is diluted with methanol (200 mL) and K2CO3 (35 g) is added. After 1 hour, the mixture is concentrated and diluted with water and the solid is collected by filtration to provide T-5. |
| 3.1 g |
With pyridine; In dichloromethane; at 80℃; for 16h; |
To a solution of 5-bromo-2-methylpyridin-3-amine (3.2 g, 17. mmol) in dichloromethane (100 mL) was added pyridine (2.03 g, 25.7 mmol) and acetic anhydride (2.63 g, 25.7 mmol). The mixture was stirred at 80 C for 16 h. The mixture was poured intowater (80 mL) and extracted with dichloromethane (3 x 150 mL). The organic layer was concentrated in vacuo, and the resultant residue was purified by flash column chromatography (15 -30% ethyl acetate in petroleum ether) to afford N-(5-bromo-2-methylpyridin-3-yl)acetamide (3.1 g, 80% yield). 1H NMR (400 MHz, Chloroform-d) oe: 8.48 (s, 1 H), 8.32 (s, 1 H), 7.09 (br s. 1 H), 2.45 (s, 3 H), 2.23 (s, 3 H). |
| 3.1 g |
With pyridine; In dichloromethane; at 80℃; for 16h;Inert atmosphere; |
To a solution of 5-bromo-2-methylpyridin-3-amine (3.2 g, 17 mmol) in dichloromethane (100 mL) was added pyridine (2.03 g, 25.7 mmol) and acetic anhydride (2.63 g, 25.7 mmol). The mixture was stirred at 80 C. for 16 h. The mixture was poured into water (80 mL) and extracted with dichloromethane (3*150 mL). The organic layer was concentrated in vacuo, and the resultant residue was purified by flash column chromatography (15?30% ethyl acetate in petroleum ether) to afford N-(5-bromo-2-methylpyridin-3-yl)acetamide (3.1 g, 80% yield). 1H NMR (400 MHz, Chloroform-d) delta 8.48 (s, 1H), 8.32 (s, 1H), 7.09 (br s. 1H), 2.45 (s, 3H), 2.23 (s, 3H). |
|
With triethylamine; In dichloromethane; at 20℃; for 24h; |
[0005701 To a stirred solution ofcompound4 (1.15 g, 1 eq) in DCM (10 mL), acetic anhydride (0.99 mL,1.7 eq) and TEA (1 mL, 2.3 eq) were added and stirred at room temperature for 24 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was quenched with saturated sodium bicarbonate solution and extracted with ethyl acetate (3 X 25 mL). Combined organic extracts were dried over anhydrous sodium sulfate and evaporated under reduced pressure to afford the title compound 5. LCMS (mlz):231.00 (M+2). |
|
With triethylamine; In dichloromethane; at 150℃; for 12h; |
[0260] [0261] [A] N- (5-Bromo-2-methylpyridin-3-yl) acetamide [0262] [0263] To a solution of 5-bromo-2-methylpyridin-3-amine (20 g, 106 mmol) and Et3N (11.7 g, 116 mmol) in DCM (200mL) was added Ac2O (14.1 g, 139 mmol) and the resulting reaction mixture was stirred at 150 for 12 h. Afterwards, the mixture solution was concentrated under reduced pressure. The residule was diluted with water, basified with aq. NaHCO3 solution (10 mL), and then extracted with EtOAc (10 mL x 3). The combined organic layers were dried over anhy. Na2SO4, filtered, and concentrated in vacuo to giv a crude title compound (14 g, 66.3yield) as a solid. MS 231.7 [M+H] +. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping