| 90% |
|
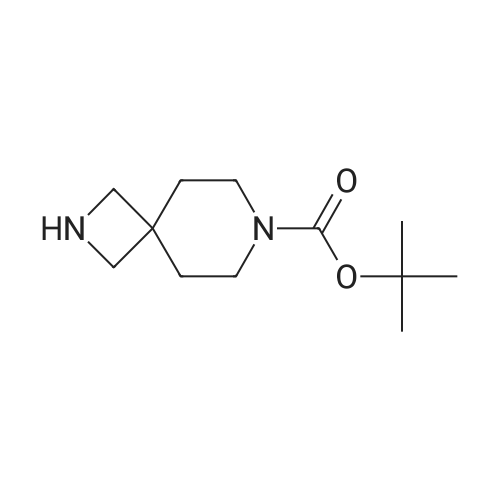
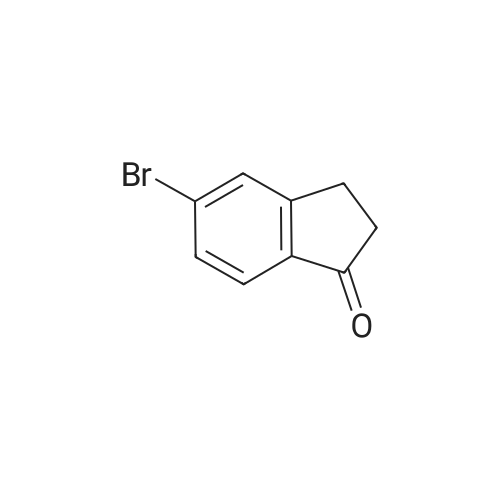
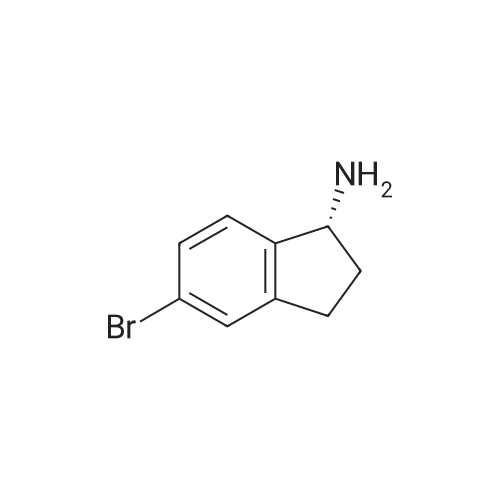
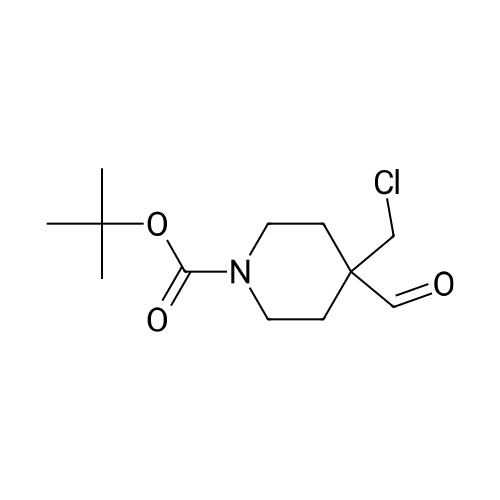
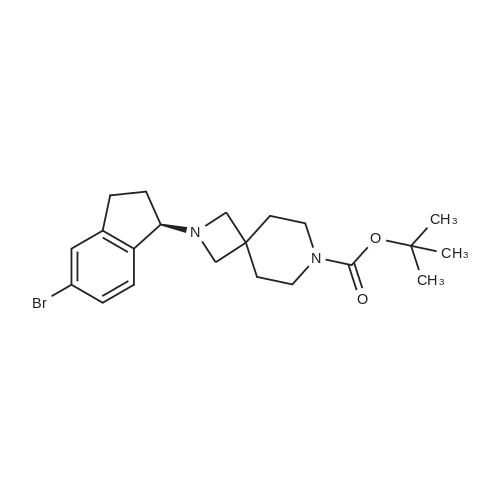
To a solution of <strong>[1228561-27-0](1R)-5-bromoindan-1-amine</strong> (SM-1, 1835 g, 8.66 mol) in anhydrous methanol (24 L) was added tert-butyl 4-(chloromethyl)-4-formylpiperidine-1-carboxylate (SM-3a, 2310 g, 8.83 mol). The mixture was stirred at 50 C. for 16 h, and cooled to rt. Sodium cyanoborohydride (1000 g, 15.9 mol) in THF (15 L) was added via a syringe pump over 2 hours. The mixture was stirred at 60 C. for 24 hours under nitrogen with a vent into a bleach bath. The reaction was cooled to 20 C. and transferred via a cannula into a vessel containing 24 L of 2.5M sodium hydroxide, and 30 L of DCM. The layers were separated and the aqueous layer was extracted with DCM (2×5 L). The aqueous layer was treated to destruct residual sodium cyanoborohydride. The combined organic layers were dried (MgSO4) and concentrated under reduced pressure. The crude material was slurried in MTBE (7 L) by stirring at 40 C. for 1 h and at rt for 1 h. The solid was filtered, and washed with MTBE (2×500 mL) and dried under vacuum oven at 50 C. to give the title product as a white crystals (3657 g, 90%). MS (ES+) 422.3 (M+H)+. 1H NMR (CDCl3) delta 1.44 (s, 9H), 1.67 (dd, 4H), 1.84-1.93 (m, 1H), 2.07-2.16 (m, 1H), 2.72-2.81 (m, 1H), 2.95-3.15 (m, 5H), 3.31 (dd, 4H), 3.85 (br s, 1H), 7.12 (d, 1H), 7.28 (br s, 1H), 7.35 (br s, 1H). [alpha]D20=+39.6 deg (c=1.06 mg/mL, MeOH). |
| 90% |
|
To a solution of <strong>[1228561-27-0](R)-5-bromo-2,3-dihydro-1H-inden-1-amine</strong> (1835 g, 8.66 mol) inanhydrous methanol (24 L) was added tert-butyl 4-(chloromethyl)-4-formylpiperidine-1-carboxylate (2310 g, 8.83 mol). The mixture was stirred at 50C for 16 h, and cooled to rt. Sodium cyanoborohydride (1000 g, 15.9 mol) in THF (15 L) was added via a syringe pump over 2 hours. The mixture was stirred at 60C for 24 hours under nitrogen with a vent into a bleach bath. The reaction was cooled to 20C and transferred via a cannulainto a vessel containing 24 L of 2.5M sodium hydroxide, and 30 L of DCM. The layers were separated and the aqueous layer was extracted with DCM (2 x 5 L). The aqueous layer was treated to destruct residual sodium cyanoborohydride. The combined organic layers were dried (Mg504) and concentrated under reduced pressure. The crude material was slurried in MTBE (7 L) by stirring at 40C for 1 h and at rt for 1 h. The solidwas filtered, and washed with MTBE (2 x 500 mL) and dried under vacuum oven at50C to give the title product as a white crystals (3657 g, 90%). MS (ES+) 422.3(M+H). 1H NMR (CDCI3) 1.44 (s, 9H), 1.67 (dd, 4H), 1.84-1.93 (m, 1H), 2.07-2.16 (m,1H), 2.72-2.81 (m,1 H), 2.95-3.15 (m, 5H), 3.31 (dd, 4H), 3.85 (brs, 1H), 7.12 (d, 1H),7.28 (br s, 1 H), 7.35 (br s, 1 H). [a] = +39.6 deg (c = 1.06 mgmL, MeOH). |
| 74% |
|
To a solution of <strong>[1228561-27-0](R)-5-bromoindan-1-amine</strong> (1835 g, 8.66 mol) in anhydrous methanol (24 L) was added tert-butyl 4-(chloromethyl)-4-formylpiperidine-1-carboxylate (2310 g, 8.83 mol). The mixture was stirred at 50 C for 16 h, and cooled to rt. Sodium cyanoborohydride (1000 g, 15.9 mol) in THF (15 L) was added via a syringe pump over 2 h. The mixture was stirred at 60 C for 24 h under nitrogen with a vent into a bleach bath. The reaction was cooled to 20 C and transferred via a cannula into a vessel containing 24 L of 2.5M sodium hydroxide, and 30 L of DCM. The layers were separated and the aqueous layer was extracted with DCM (2 x 5L). The aqueous layer was treated to destruct residual sodium cyanoborohydride. The combined organic layers were dried (MgSO4) and concentrated under reduced pressure. The crude material was slurried in methyl tert-butyl ether (7 L) by stirring at 40 C for 1 h and at rt for 1 h. The solid was filtered, and washed with methyl tert-butyl ether (2 x 500 mL) and dried in a vacuum oven at 50 C to give 3 (2699 g, 74%) as white crystals. 1H NMR (500 MHz, Cd3Od): delta 7.42 (s, 1 H), 7.32 (d, J = 10 Hz, 1 H), 7.26 (d, J = 10.0 Hz, 1 H), 4.01-3.97 (m, 1 H), 3.36 (s, 4 H), 3.28-3.30 (m, 2 H), 3.24 (d, J = 10.0 Hz, 2 H), 3.09-3.03 (m, 1 H), 2.86-2.80 (m, 1 H), 2.25-2.18 (m, 1 H), 1.92-1.86 (m, 1 H), 1.72-1.70 (m, 4 H), 1.45 (s, 9 H); 13C NMR (125 MHz, Cd3Od): delta 155.3, 147.3, 142.7, 129.1, 127.9, 126.1, 119.2, 80.0, 71.3, 62.2, 51.5, 35.5, 33.8, 29.9, 29.1, 27.5; HRMS: m/z 421.1480 (calc 421.1485 for C21H29BrN2O2+ H); [alpha]20D= +39.6 deg (c = 1.06 mg/mL, MeOH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping