| 100% |
|
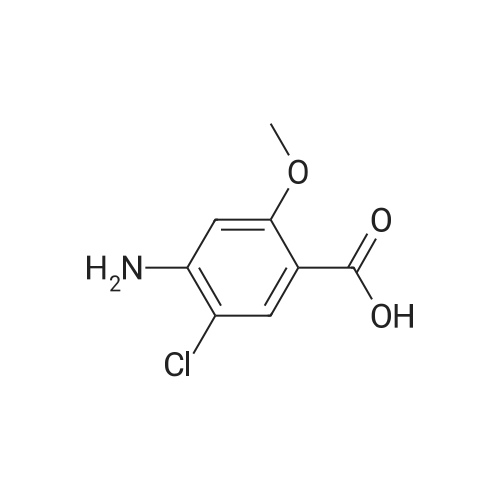
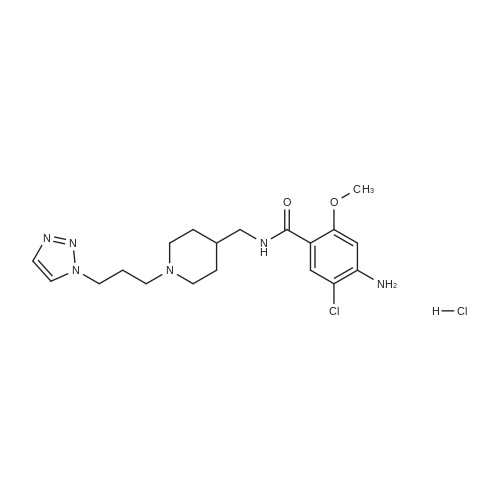
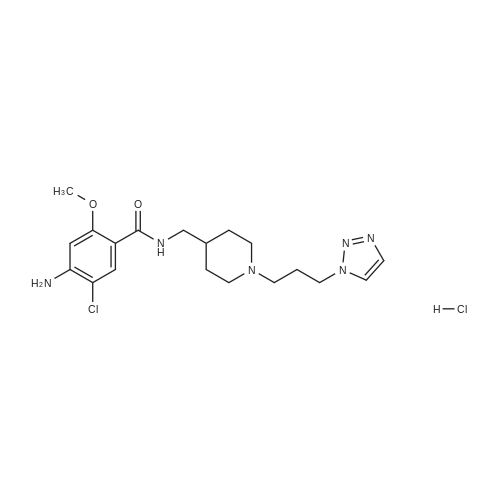
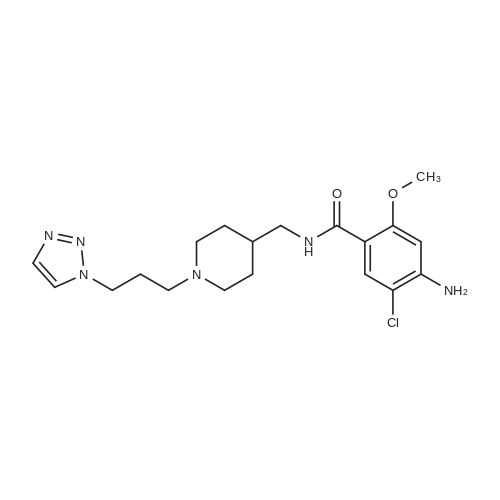
(0173) 4-Amino-5-chloro-2-methoxybenzoic acid (0.20 g) was dissolved in acetonitrile (10 mL). Carbonyldiimidazole (0.19 g) was added to the solution and stirred at 19-22 C. for 4 hours. After triethylamine (0.20 g) and [1-{3-(1H-1,2,3-triazol-1-yl)propyl}piperidin-4-yl]methanamine (0.27 g) were sequentially added thereto, the temperature of the resulting solution was elevated. Then the solution was stirred under reflux. After 21 hours of stirring, water (10 mL) was added thereto, stirred for 5 minutes, and layers were separated. Dichloromethane (10 mL) was added to an aqueous layer and an organic layer was extracted. Water (10 mL) and 1N hydrochloric acid (4 mL) were added to the collected organic layers to extract the aqueous layer. 2N sodium hydroxide (about 4 mL) was added to the aqueous layer to adjust pH of about 10. The extraction was performed twice with a mixed solvent of dichloromethane (8 mL) and 2-propanol (2 mL). The collected organic layers were dried with anhydrous magnesium sulfate and filtered, and then washed with dichloromethane (10 mL). The solvent was removed by concentrating the filtered organic solution under reduced pressure. The resulting solids were dried under reduced pressure at 19-22 C. for 17 hours to obtain the titled compound (0.41 g; yield: 100%). (0174) 1H NMR (400 MHz, CDCl3) delta 8.08 (s, 1H), 7.72 (m, 1H), 7.66 (s, 1H), 7.54 (s, 1H), 6.27 (s, 1H), 4.43 (t, 2H), 4.36 (s, 1H), 3.88 (s, 3H), 3.30 (t, 2H), 2.83 (d, 2H), 2.27 (t, 2H), 2.06 (t, 2H), 1.90 (t, 2H), 1.70 (m, 2H), 1.58 (m, 1H), 1.29 (m, 2H) |
| 100% |
|
4-Amino-5-chloro-2-methoxybenzoic acid (0.20 g) was dissolved in acetonitrile (10 mL). Carbonyldiimidazole (0.19 g) was added to the solution and stirred at 19-22 C. for 4 hours. After triethylamine (0.20 g) and [1-{3-(1H-1,2,3-triazol-1-yl)propyl}piperidin-4-yl]methanamine (0.27 g) were sequentially added thereto, the temperature of the resulting solution was elevated. Then the solution was stirred under reflux. After 21 hours of stirring, water (10 mL) was added thereto, stirred for 5 minutes, and layers were separated. Dichloromethane (10 mL) was added to an aqueous layer and an organic layer was extracted. Water (10 mL) and 1N hydrochloric acid (4 mL) were added to the collected organic layers to extract the aqueous layer. 2N sodium hydroxide (about 4 mL) was added to the aqueous layer to adjust pH of about 10. The extraction was performed twice with a mixed solvent of dichloromethane (8 mL) and 2-propanol (2 mL). The collected organic layers were dried with anhydrous magnesium sulfate and filtered, and then washed with dichloromethane (10 mL). The solvent was removed by concentrating the filtered organic solution under reduced pressure. The resulting solids were dried under reduced pressure at 19-22 C. for 17 hours to obtain the titled compound (0.41 g; yield: 100%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping