| 38% |
With potassium carbonate In water; acetone at 0 - 20℃; for 18h; |
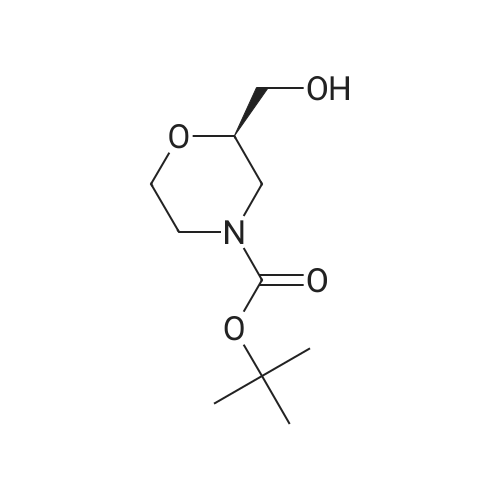
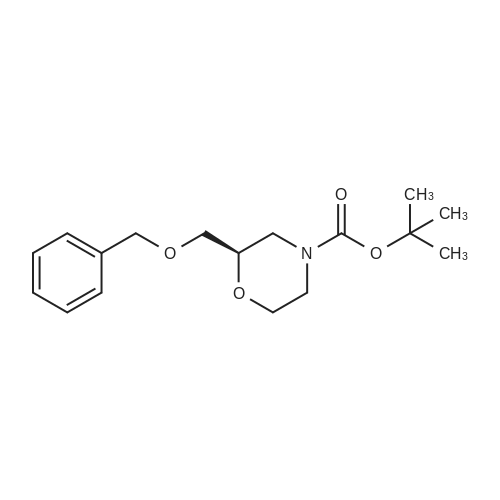
111 tert-butyl (2R)-2-[(benzyloxy)methyl]morpholine-4-carboxylate
A so[ution of Intermediate 110 in acetone (400 mL) and water (120 mL) was coo[ed to 0 °C and potassium carbonate (70 g, 0.5 mo[) was added fo[[owed by di-tertbuty[ dicarbonate (44 g, 0.2 mo[). The reaction mixture was a[[owed to warm to ambient temperature and was stirred for 18 h. Acetone was removed under reduced pressure and the aqueous solution extracted twice with EtOAc. The combined organics were dried (MgSO4), filtered and concentrated under reduced pressure. The crude material was purified by Biotage IsoleraTM chromatography(eluting with 0 - 25 % EtOAc in heptane on a pre-packed 340 g silica gel column) to give 19.8 g (38% yield) of the title compound as pale yellow oil.1H NMR (250 MHz, chloroform-d): 6 [ppm] 7.39 - 7.27 (m, 5H), 4.56 (5, 2H), 4.03 -3.73 (m, 3H), 3.69- 3.34 (m, 4H), 3.05-2.86 (m, 1H), 2.84- 2.65 (m, 1H), 1.46 (5,9H).LC-MS (Method A) Rt =1.27 mm, MS (ESipos): m/z = 252 (M-tBu). |
|
With triethylamine In dichloromethane |
3.b 3(b)
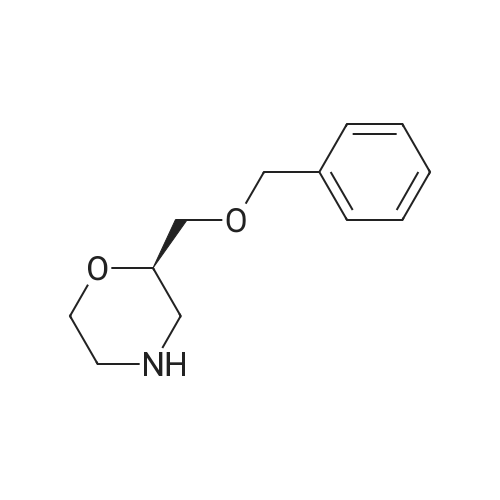
3(b) (R)-2-(Benzyloxymethyl)-4-t-butoxycarbonylmorpholine 7.3 ml of triethylamine were added to 100 ml of methylene chloride containing 10.9 g of (R)-2-(benzyloxymethyl)morpholine [prepared as described in step (a) above], and then 20 ml of methylene chloride containing 12.1 g of di-t-butyl pyrocarbonate was added dropwise to the mixture on an ice bath. The mixture was then stirred for 2 hours at room temperature, after which the reaction mixture was condensed by evaporation under reduced pressure. The residue was purified by silica gel column chromatography using a 1: 4 by volume mixture of ethyl acetate and hexane as the eluent, to obtain 14. 2 g of the title compound as a syrup. Optical rotation [α] [25/D ] - 17.2° (c = 1, chloroform). Nuclear Magnetic Resonance Spectrum (CDCl3), δ ppm: 1.48 (9H, singlet); 2.5 - 4.05 (9H, multiplet); 4.52 (2H, singlet); 7.32 (5H, singlet). |
|
In 1,4-dioxane; water at 20℃; for 5h; |
2.a
Step a) 2R-Benzyloxymethyl-morpholine-4-carboxylic acid tert-butyl ester2g of S-(+)-2-(benzyloxymethyl)-oxirane and 7 g of 2-aminoethyl hydrogen sulfate were weighed into a 100 ml round bottle flask, 2 g of NaOH dissolved in H2O was added and the stirred mixture was heated at 50 0C for 1 hour. 4 g of NaOH dissolved in 10 ml H2O, solution was added to the stirred mixture, which was then heated at 55 0C for 16 h. After cooling the mixture to room temperature, it was diluted with 100 ml H2O and 100 ml dioxane and 2.66 g of di-tert-butyl dicarbonate was added. The mixture was stirred at room temperature for 5 hours, transferred into a separation funnel and extracted with 2 x 75 ml of toluene. Combined organic phases were washed with 2 x 50 ml of 1 M citric acid (aq.), once with brine, dried over Na2SO4 and concentrated in vacuo. The crude material was purified on silica yielding 2R- Benzyloxymethyl-morpholine-4-carboxylic acid tert-butyl ester 1.85 g (49%) as a clear oil. |
|
With triethylamine In dichloromethane for 16h; |
1.2
Step 2: (R)-2-(Benzyloxymethyl)morpholine-4-carboxylic acid tert-butyl ester: To a stirred solution of (R)-2-(benzyloxymethyl)morpholine (Stepl) (16.0 g) and triethylamine (12.9 mL) in DCM (230 mL) was added di-tert-butyl dicarbonate (18.5 g). After 16 h, aqueous sodium bicarbonate (200 mL) was added and the mixture was extracted into DCM (100 mL x 3). The organic layers were combined, washed with brine and dried over magnesium sulfate, filtered and concentrated. The resulting oil was chromatographed (SiO2: eluent 1 : 5 ethyl acetate/40-60 petroleum ether) to yield the subtitle compound as a colourless oil (12.1 g).1H NMR δ(CDCl3): 1-46 (9H, s), 2.74 (IH, t), 2.95 (IH, t), 3.42 - 3.65 (4H, m), 3.79 3.97 (3H, m), 4.56 (2H, s), 7.24 - 7.39 (5H, m). |
|
With potassium carbonate In water; acetone at 0 - 20℃; for 0.5h; |
9.2
To a solution of crude (i?)-2-(benzyloxymethyl)morpholine (~14 g) in acetone (100 mL) and H2O (30 mL) at O0C, there was added K2CO3 (25.2 g, 182.7 mmol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (~30 min). Acetone was removed under vacuum, and the aqueous solution was extracted with CH2Cl2 (4 x 10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)-tert- butyl 2-(benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400MHz, CDCl3): 7.34 (m, 5 H), 4.56 (s, 2 H), 3.88 (d, 2 H), 3.82 (br, 1 H), 3.40 (m, 1 H), 3.48 (m, 3 H), 2.94 (m, 1 H), 2.76 (m, 1 H), 1.44 (s, 9 H); MS m/z 330 (M+Na+). |
|
With potassium carbonate In water; acetone at 0 - 20℃; |
3.2
Step 2. (R)-tert-Butyl 2-(benzyloxymethyl)morpholine-4-carboxylate To a solution of crude (R)-2-(benzyloxymethyl)morpholine (~14 g) in acetone (100 mL) and H2O (30 mL) at 0° C., was added K2CO3 (25.2 g, 182.7 mmol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (~30 min). The acetone was removed under vacuum and the aqueous solution was extracted with CH2Cl2 (4*10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)-tert-butyl 2-(benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400 MHz, CDCl3): δ=7.34 (m, 5H), 4.56 (s, 2H), 3.88 (d, 2H), 3.82 (br, 1H), 3.40 (m, 1H), 3.48 (m, 3H), 2.94 (m, 1H), 2.76 (m, 1H), 1.44 (s, 9H); MS m/z 330 (M+Na+). |
|
In 1,4-dioxane; water at 20℃; for 5h; |
10.1
2g of S-(+)-2-(benzyloxymethyl)-oxirane and 7 g of 2-aminoethyl hydrogen sulfate were weighed into a 100 ml round bottle flask, 2 g of NaOH dissolved in H2O was added and the stirred mixture was heated at 50 0C for 1 hour. 4 g of NaOH dissolved in 10 ml H2O, solution was added to the stirred mixture, which was then heated at 55 0C for 16 h. After cooling the mixture to room temperature, it was diluted with 100 ml H2O and 100 ml dioxane and 2.66 g of di-tert-butyl dicarbonate was added. The mixture was stirred at room temperature for 5 hours, transferred into a separation funnel and extracted with 2 x 75 ml of toluene. Combined organic phases were washed with 2 x 50 ml of 1 M citric acid (aq.), once with brine, dried over Na2SO4 and concentrated in vacuo. The crude material was purified on silica yielding 2R-Benzyloxymethyl-morpholine-4-carboxylic acid tert-butyl ester 1.85 g (49%) as a clear oil. |
|
With potassium carbonate In water; acetone at 0 - 20℃; for 0.5h; |
26.2
To a solution of crude (R)-2-(benzyloxymethyl)morpholine (~14 g) in acetone(100 mL) and H2O (30 mL) at O°C, there was added K2CO3 (25.2 g, 182.7 mmol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (-30 min), acetone was removed under vacuum, and the aqueous solution was extracted with CH2Cl2 (4 x 10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)-tert-butyl 2- (benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400MHz, CDCl3): 7.34 (m, 5 H), 4.56 (s, 2 H), 3.88 (d, 2 H), 3.82 (br, 1 H), 3.40 (m, 1 H), 3.48 (m, 3 H), 2.94 (m, 1 H), 2.76 (m, 1 H), 1.44 (s, 9 H); MS m/z 330 (M+Na+). |
|
With potassium carbonate In water; acetone at 0 - 20℃; |
6.2
To a solution of crude (R)-2-(benzyloxymethyl)morpholine (14 g) in acetone (100 mL) and H2O (30 mL) at 0° C., there was added K2CO3 (25.2 g, 182.7 mmol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (30 min). Acetone was removed under vacuum, and the aqueous solution was extracted with CH2Cl2 (4×10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)-tert-butyl 2-(benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400 MHz, CDCl3) δ ppm 7.34 (m, 5H), 4.56 (s, 2H), 3.88 (d, 2H), 3.82 (br, 1H), 3.40 (m, 1H), 3.48 (m, 3H), 2.94 (m, 1H), 2.76 (m, 1H), 1.44 (s, 9H); MS m/z 330 (M+Na+). |
|
With potassium carbonate In water; acetone at 0 - 20℃; for 0.5h; |
|
|
With potassium carbonate In water; acetone at 0 - 20℃; |
10.2
Step 2. (R)-tert-Buty 2-(benzyloxymethyl)morpholine-4-carboxylate: To a solution of crude (i?)-2-(benzyloxymethyl)morpholine (~14 g) in acetone (100 rnL) and H2O (30 rnL) at O0C, there was added K2CO3 (25.2 g, 182.7 mmol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (~30 min). Acetone was removed under vacuum, and the aqueous solution was extracted with CH2Cl2 (4 x 10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)-tert-buty{ 2- (benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400MHz, CDCl3): 7.34 (m, 5 H), 4.56 (s, 2 H), 3.88 (d, 2 H), 3.82 (br, 1 H), 3.40 (m, 1 H), 3.48 (m, 3 H), 2.94 (m, 1 H), 2.76 (m, 1 H), 1.44 (s, 9 H); MS m/z 330 (M+Na+). |
|
With potassium carbonate In water; acetone at 0℃; for 2h; Inert atmosphere; |
6.1.32. Tert-butyl (R)-2-((benzyloxy)methyl)morpholine-4-carboxylate (30)
Benzyl-(R)-glycidyl ether (2.00 g, 12.2 mmol) and NaOH (4.00 g,100 mmol) in H2O (9.2 mL) and MeOH (3.6 mL) were treated with2-aminoethyl hydrogen sulphate (7.00 g, 49.59 mmol). The reactionmixturewas stirred for 90 min at 40 °C. The mixturewas allowed tocool to room temperature, toluene (14 mL) and NaOH (2.00 g,50.0 mmol) were added and then it was stirred overnight at 65 °C.Toluene (5 mL) and H2O (20 mL) were added and the organic layerwas separated. The water layer was extracted with CH2Cl2(2 10 mL). The combined organic layers were dried over Na2SO4,filtered and concentrated in vacuo to give crude amine which wastaken up in acetone (20 mL) and H2O (6 mL) at 0 °C. Di-tert-butyldicarbonate (2.60 g, 11.9 mmol) was added and the resultingmixture was stirred vigorously for 2 h. The acetone was removedunder reduced pressure and the aqueous solution was extractedwith CH2Cl2. The organic layer was washed with brine, dried overNa2SO4, filtered and concentrated in vacuo. FCC (hexane/EtOAc10:0 → 7:3) afforded the title compound as a colorless oil in 43%(over 2 steps). 1H NMR (300 MHz, 80 °C, DMSO-d6) δ ppm 1.41 (s,9 H) 2.69 (dd, J 12.9, 9.4 Hz, 1 H) 2.87 (ddd, J 13.2, 11.4, 3.5 Hz,1 H) 3.28e3.56 (m, 4 H) 3.68 (ddt, J 13.2, 3.0, 1.6 Hz, 1 H)3.75e3.86 (m, 2 H) 4.50 (s, 2 H) 7.22e7.38 (m, 5 H). 13C NMR(75 MHz, 80 °C, DMSO-d6) δ ppm 27.7, 43.0, 45.3, 65.1, 70.2, 72.2,73.6, 78.7, 126.9, 127.0, 127.7, 138.0, 153.6. HRMS (ESI-TOF) m/z:[M+H]+ Calcd for C17H26NO4 308.18563; Found 308.1867. |
|
With potassium carbonate In water; acetone at 0 - 20℃; for 0.5h; |
34.2; 36.2
To a solution of crude (R)-2-(benzyloxymethyl)morpholine (~14 g) in acetone (100 mL) and H2O (30 mL) at O0C, was added K2CO3 (25.2 g, 182.7 ramol), followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (~30 min). Acetone was removed under vacuum and the aqueous solution was extracted with CH2Cl2 (4 x 10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)- •tert-butyl 2-(benzyloxymelhyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400MHz3 CDCl3): 7.34 (m, 5 H), 4.56 (s, 2 H), 3.88 (d, 2 H), 3.82 (br, 1 H)5 3.40 (m, 1 H), 3.48 (m, 3 H)5 2.94 (m, 1 H), 2.76 (m, 1 H), 1.44 (s, 9 H); MS m/z 330 (M+Na+). |
|
With potassium carbonate In water; acetone at 0 - 20℃; for 0.5h; |
4.2
Step 2. (R)-tert-Butyl 2-(benzyloxymethyl)morpholine-4-carboxylate. To a solution of crude (R)-2-(benzyloxymethyl)morpholine (~14 g) in acetone (100 mL) and H2O (30 mL) at O0C5 was added K2CO3 (25.2 g, 182.7 mmol), • followed by (Boc)2O (14.6 g, 67.0 mmol). The resulting solution was warmed to rt, and stirred until no starting material remained (~30 min). Acetone was removed under vacuum and the aqueous solution was extracted with CH2Cl2 (4 x 10 mL). The combined organic layers were washed with H2O (10 mL) and the solvent was removed. The residue was purified by flash column chromatography to give (R)- rer/-butyl 2-(benzyloxymethyl)morpholine-4-carboxylate (8.33 g, 44% over 2 steps). 1H NMR (400MHz, CDCl3): 7.34 (m, 5 H)5 4.56 (s, 2 H), 3.88 (d5 2 H), 3.82 (br, 1 H), 3.40 (m, 1 H), 3.48 (m, 3 H), 2.94 (m, 1 H), 2.76 (m, 1 H), 1.44 (s, 9 H); MS m/z 330 (M+Na+). . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping