| 75% |
With caesium carbonate In N,N-dimethyl-formamide at 120℃; for 4h; Sealed tube; |
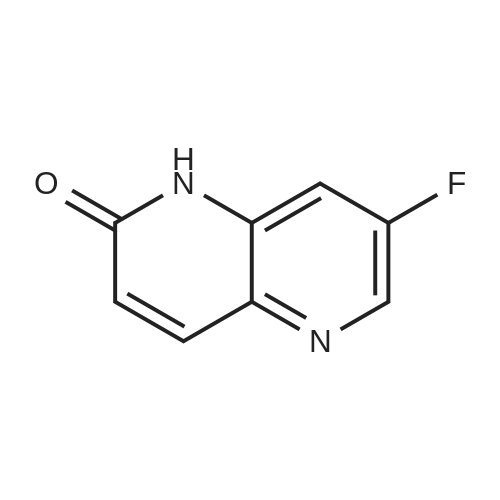
4.1.3 General procedure for synthesis of 7-fluoro-1-(2-hydroxyethyl)-1,5-naphthyridin-2(1H)-one (7)
To a solution corresponding 7-fluoro-1,5-naphthyridin-2(1H)-one (6) (1g, 6.09 mmol) in dry N,N′-dimethyl formamide (10 mL) was added Cs2CO3 (4.95 g, 15.23 mmol) and followed by 2-bromoethanol (0.913 g, 7.31 mmol) and heated in sealed tube at 120°C for 4h (monitored by TLC & LCMS for completion). The reaction mixture was then filtered through celite and washed with dichloromethane. The filtrate was concentrated under reduced pressure. The reaction mixture was further extracted with ethyl acetate (3 × 40 mL). The combined organic extracts were washed with brine (2 × 40 mL) and water (3 × 50 mL), dried (MgSO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using hexane: ethyl acetate as eluent to give the corresponding 7-fluoro-1-(2-hydroxyethyl)-1,5-naphthyridin-2(1H)-one (7) (0.953 g, 75 %) as pale yellow solid. M.p: 241-243°C. 1H NMR [300MHz, DMSO-d6] δH: 8.14-6.52 (m, 4H), 3.74-3.47 (m, 5H). 13C NMR [DMSO-d6] δC: 162.3, 157.8, 147.5, 140.1, 132.3, 125.6, 123.2, 110.1, 64.5, 46.2. ESI-MS m/z 209 (M+H)+. Anal Calcd for C10H9FN2O2: C, 57.69; H, 4.36; N, 13.46; Found: C, 57.72; H, 4.38; N, 13.45. |
| 75.6% |
With caesium carbonate In N,N-dimethyl-formamide for 4h; Sealed tube; |
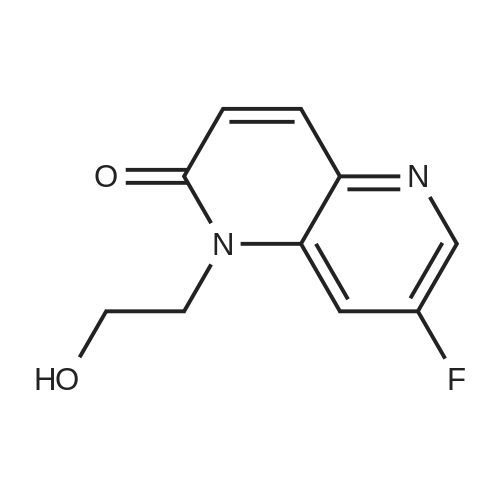
General procedure for synthesis of 7-fluoro-1-(2-hydroxyethyl)-1,5-naphthyridin-2(1H)-one (2)
To a solution of corresponding 7-fluoro-1,5-naphthyridin-2(1H)-one (1) (1 g, 6.09 mmol) (1 equiv) in dry N,N' dimethyl formamide (10 mL) was added Cs2CO3 (4.95 g, 15.23 mmol) (2.5 equiv) and followed by 2-bromoethanol (0.913 g, 7.31 mmol) (1.2 equiv) and heated in sealed tube at 120 °C for 4 h (monitored by TLC & LCMS for completion). The reaction mixture was then filtered through celite and washed with dichloromethane. The filtrate was concentrated under reduced pressure. The reaction mixture was further extracted with ethyl acetate (3 * 40 mL). The combined organic extracts were washed with brine (2 * 40 mL) and water (3 * 50 mL), dried (MgSO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using hexane (40%)/ethyl acetate (60%) as eluent to give the corresponding 7-fluoro-1-(2-hydroxyethyl)-1,5-naphthyridin-2(1H)-one (0.953 g, 75.6%) as pale yellow solid. 1H NMR [300 MHz, DMSO-d6] δH: 3.68 (t, J = 6 Hz, 2H, -CH2), 4.28 (t, J = 6 Hz, 2H, -CH2), 6.67 (d, J = 9.6 Hz, 1H, -C=CH), 7.51 (s, 1H, Ar-H), 7.86 (d, J = 9.6 Hz, 1H, -C=CH), 8.13 (s, 1H, -OH), 8.37 (d, J = 2.6 Hz, 1H, Ar-H). 13C NMR [DMSO-d6] δc: 162.3, 157.8, 147.5, 140.1, 132.3, 125.6, 123.2, 110.1, 64.5, 46.2. ESI-MS m/z (Calcd for C10H9FN2O2: 208.19); Found: 209.24 (M+H)+. Anal Calcd for C10H9FN2O2: C, 57.69; H, 4.36; N, 13.46; Found: C, 57.72; H, 4.38; N, 13.45. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping