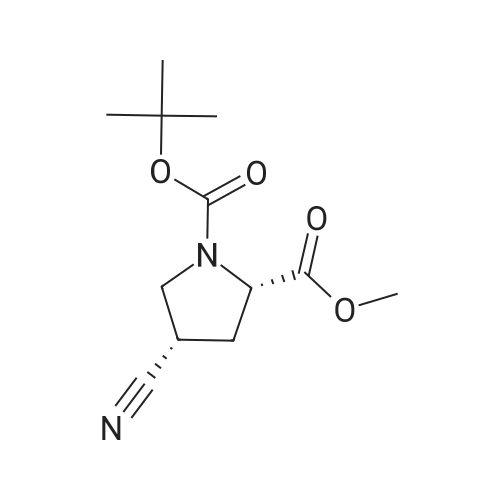
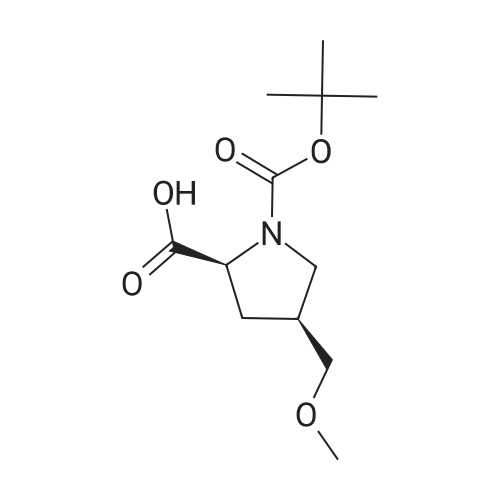
Alternatived Products of [ 1378388-35-2 ]
Product Details of [ 1378388-35-2 ]
CAS No. : 1378388-35-2
MDL No. : MFCD28501727
Formula :
C12 H19 NO6
Boiling Point : -
Linear Structure Formula : -
InChI Key : HMHBAEDPNFIUEI-YUMQZZPRSA-N
M.W :
273.28
Pubchem ID : 69673205
Synonyms :
Calculated chemistry of [ 1378388-35-2 ]
Physicochemical Properties
Num. heavy atoms : 19
Num. arom. heavy atoms : 0
Fraction Csp3 : 0.75
Num. rotatable bonds : 6
Num. H-bond acceptors : 6.0
Num. H-bond donors : 1.0
Molar Refractivity : 69.26
TPSA : 93.14 Ų
Pharmacokinetics
GI absorption : High
BBB permeant : No
P-gp substrate : No
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : No
CYP2D6 inhibitor : No
CYP3A4 inhibitor : No
Log Kp (skin permeation) : -7.45 cm/s
Lipophilicity
Log Po/w (iLOGP) : 2.11
Log Po/w (XLOGP3) : 0.73
Log Po/w (WLOGP) : 0.49
Log Po/w (MLOGP) : 0.41
Log Po/w (SILICOS-IT) : -0.13
Consensus Log Po/w : 0.72
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 0.0
Bioavailability Score : 0.56
Water Solubility
Log S (ESOL) : -1.6
Solubility : 6.89 mg/ml ; 0.0252 mol/l
Class : Very soluble
Log S (Ali) : -2.26
Solubility : 1.49 mg/ml ; 0.00544 mol/l
Class : Soluble
Log S (SILICOS-IT) : -0.21
Solubility : 167.0 mg/ml ; 0.611 mol/l
Class : Soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 1.0 alert
Leadlikeness : 0.0
Synthetic accessibility : 3.44
Safety of [ 1378388-35-2 ]
Application In Synthesis of [ 1378388-35-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 1378388-35-2 ]
1
[ 487048-28-2 ]
[ 1378388-35-2 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1.1: hydrogenchloride / 1,4-dioxane / 16 h / 20 °C
1.2: 12 h / 20 °C
2.1: water; sodium hydroxide / tert-Amyl alcohol / 5.25 h / 0 °C / Cooling with ice
Multi-step reaction with 2 steps
1.1: hydrogenchloride / 1,4-dioxane; methanol / 16 h / 20 °C
1.3: 12 h / 20 °C
2.1: sodium hydroxide; water / tetrahydrofuran / 5 h / 0 °C
Multi-step reaction with 2 steps
1.1: hydrogenchloride / 1,4-dioxane / 16 h / 20 °C
1.2: 12 h / 20 °C
2.1: sodium hydroxide; water / tetrahydrofuran / 5 h / 0 °C
2
[ 1378388-35-2 ]
[ 1377049-84-7 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 11 steps
1: triethylamine / tetrahydrofuran / 0.75 h / 0 °C / Cooling with ice
2: water; sodium tetrahydroborate / tetrahydrofuran / 2 h / 0 °C / Cooling with ice
3: silver trifluoromethanesulfonate; 2,6-di-tert-butyl-pyridine / dichloromethane / 3 h / 0 - 20 °C / Cooling with ice
4: water; lithium hydroxide / tetrahydrofuran; methanol / 2 h / 20 °C
5: caesium carbonate / 4 h / 20 °C
6: pyridinium hydrobromide perbromide / dichloromethane; methanol / 1.75 h / 20 °C
7: caesium carbonate / 20 h / 50 °C
8: ammonium acetate / toluene; 2-methoxy-ethanol / 4.5 h / 110 °C
9: manganese(IV) oxide / dichloromethane / 13 h
10: hydrogenchloride / dichloromethane; 1,4-dioxane / 1 h / 20 °C
11: N-ethyl-N,N-diisopropylamine; 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate / N,N-dimethyl-formamide / 2 h / 20 °C
Multi-step reaction with 9 steps
1.1: chloroformic acid ethyl ester; triethylamine / tetrahydrofuran / 0.75 h / 0 °C
1.2: 2 h / 0 °C
2.1: 2,6-di-tert-butyl-pyridine; silver trifluoromethanesulfonate / 3 h / 0 - 20 °C
3.1: water; lithium hydroxide / methanol; tetrahydrofuran / 2 h / 20 °C
4.1: caesium carbonate / 2-methyltetrahydrofuran / 4 h / 20 °C
5.1: pyridinium hydrobromide perbromide / dichloromethane; methanol / 1.75 h / 20 °C
6.1: caesium carbonate / 2-methyltetrahydrofuran / 20 h / 50 °C
7.1: ammonium acetate / toluene; 2-methoxy-ethanol / 4.5 h / 110 °C
8.1: manganese(IV) oxide / dichloromethane / 13 h
9.1: hydrogenchloride / ethanol / 3 h / 50 °C
9.2: 2 h / 20 °C
3
[ 1378388-35-2 ]
[ 1378388-16-9 ]
Reference:
[1]Patent: WO2013/75029,2013,A1
[2]Patent: US2013/309196,2013,A1
[3]Patent: US2014/178336,2014,A1
[4]Patent: US2015/361073,2015,A1
[5]Patent: US2015/361087,2015,A1
[6]Patent: CN107540679,2018,A
[7]Patent: CN107556324,2018,A

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping