| 95% |
With [[Cu(MeCN)4]PF6]; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; triethylamine In N,N-dimethyl acetamide at 0℃; for 14h; Inert atmosphere; |
4 [Example 4] (3'R, 4'S, 5'R) -6 '' - chloro-4 '- (2-chloro-3-fluoropyridin-4-yl) -4,4-dimethyl- -1 ', 2' '- Dihydrospiro [cyclohexane-1,2'-pyrrolidin-3', 3 '-indole] -5'-carboxylate
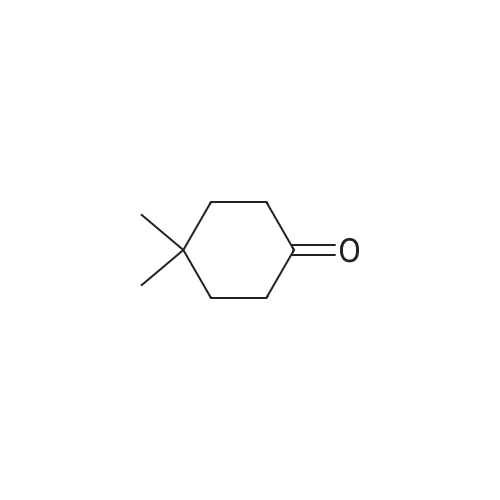
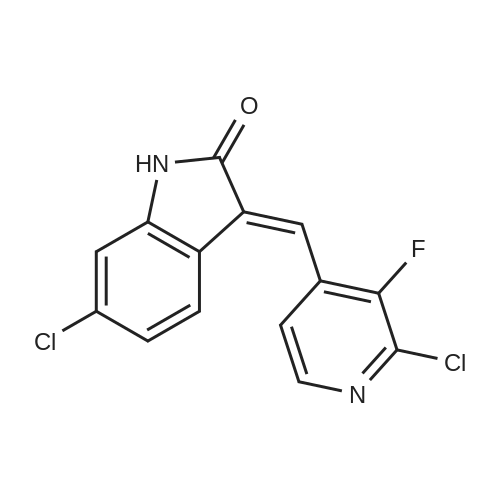
To a solution of (3E / Z) -6-chloro-3 - [(2-chloro-3-fluoropyridin-4-yl) methylene] -1,3-dihydro-2H- indole- 2-one (WO2012 / 121361) (100.7 mg), (R) -BINAP (12.1 mg, 0.019 mmol), CuOAc (2.0 mg, 0.016 mmol) was added 4,4- dimethylcyclohexanone (6.8 mL, 0.049 mmol) in N, N-dimethylacetamide (2.0 mL) was stirred at 0 ° C, and the mixture was stirred at 0 ° C for 14 hours at 0 ° C. hour. To the reaction mixture was added ethyl acetate (2 mL), water (1 mL), 20% aqueous ammonium chloride solution (1 mL), and the organic layer was vigorously stirred. The aqueous layer was extracted twice with ethyl acetate (2 mL each), all the organic layers were combined and then washed three times with water (5 mL each). |
| 80% |
With copper (I) acetate; (R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl; triethylamine In N,N-dimethyl acetamide at 0℃; for 14h; Inert atmosphere; |
4 Example 4 Ethyl (3'R,4'S,5'R)-6"-chloro-4'-(2-chloro-3-fluoropyridin-4-yl)-4,4-dimethyl-2"-oxo-1",2"-dihydrodispiro[cyclohexane-1,2'-pyrrolidine-3',3"-indole]-5'-carboxylate
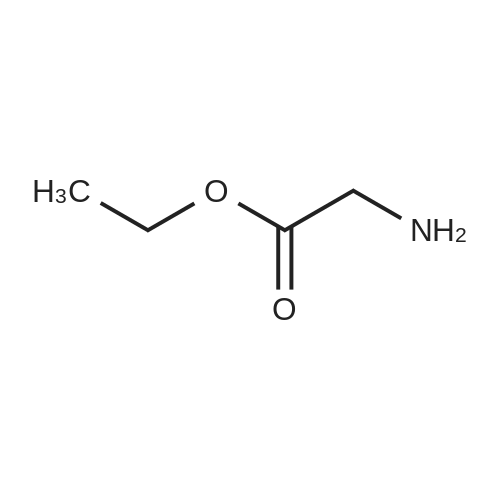
Example 4 Ethyl (3'R,4'S,5'R)-6"-chloro-4'-(2-chloro-3-fluoropyridin-4-yl)-4,4-dimethyl-2"-oxo-1",2"-dihydrodispiro[cyclohexane-1,2'-pyrrolidine-3',3"-indole]-5'-carboxylate To a mixture of (3E/Z)-6-chloro-3-[(2-chloro-3-fluoropyridin-4-yl)methylene]-1,3-dihydro-2H-indol-2-one (WO2012/121361) (100.7 mg), (R)-BINAP (12.1 mg, 0.019 mmol), and CuOAc (2.0 mg, 0.016 mmol), a solution of 4,4-dimethylcyclohexanone (61.4 mg, 0.48 mmol), glycine ethyl ester (39.5 μL, 0.39 mmol), and triethylamine (6.8 μL, 0.049 mmol) in N,N-dimethylacetamide (2.0 mL) was added under a nitrogen atmosphere, and the resulting mixture was stirred at 0° C. for 14 hours. To the reaction mixture, ethyl acetate (2 mL), water (1 mL), and a 20% aqueous ammonium chloride solution (1 mL) were added, and the mixture was vigorously stirred to separate an organic layer. The aqueous layer was subjected to extraction with ethyl acetate twice (2 mL each), and the organic layers were all combined and then washed with water three times (5 mL each). The organic layer obtained was concentrated under reduced pressure. To the residue, ethyl acetate (6 mL) and silica gel (500 mg) were added, and the silica gel was filtered off. The filtrate was concentrated under reduced pressure. To the residue, ethanol (1.0 mL) was added, then water (1 mL) was added dropwise, and the mixture was stirred overnight at room temperature. The deposited solid was filtered and dried under reduced pressure at 40° C. to obtain the title compound (134.9 mg, yield: 80%, 99% ee) as a solid. 1H NMR (500 MHz, CDCl3): δ=0.67 (s, 3H), 0.91 (s, 3H), 1.11-1.21 (m, 2H), 1.19 (t, J=7.0 Hz, 3H), 1.24-1.34 (m, 1H), 1.43-1.58 (m, 2H), 1.60-1.72 (m, 1H), 1.85-1.95 (m, 1H), 3.19 (s, 1H), 4.10-4.21 (m, 2H), 4.51 (d, J=9.0 Hz, 1H), 4.82 (d, J=9.5 Hz, 1H), 6.77 (d, J=2.0 Hz, 1H), 7.07 (dd, J=8.5, 1.5 Hz, 1H), 7.36 (dd, J=8.3, 1.8 Hz, 1H), 7.5-7.55 (m, 1H), 7.68 (bs, 1H), 8.05 (d, J=5.5 Hz, 1H). (0264) (Conditions for HPLC for Optical Purity Measurement) (0265) Column: CHIRALPAK OD-3R 4.6×150 mm, 3 μm (0266) Mobile phase: 10 mM phosphate buffer:MeCN=40:60 (0267) Flow rate: 1.0 min/min (0268) Column temperature: 40° C. (0269) Detection wavelength: 254 nm (0270) Injection quantity: 5 μL, (0271) Retention time: title compound=9.4 min, enantiomer=10.5 min |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping