| 75% |
With tetrakis(triphenylphosphine) palladium(0); In toluene; for 24h;Inert atmosphere; Reflux; |
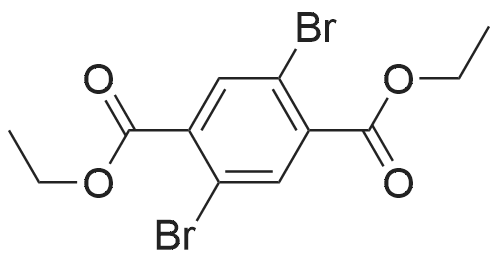
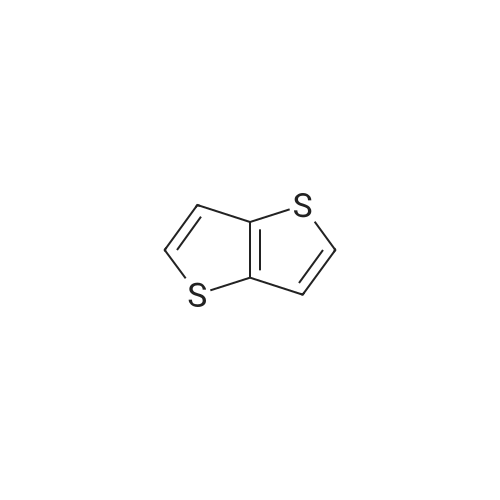
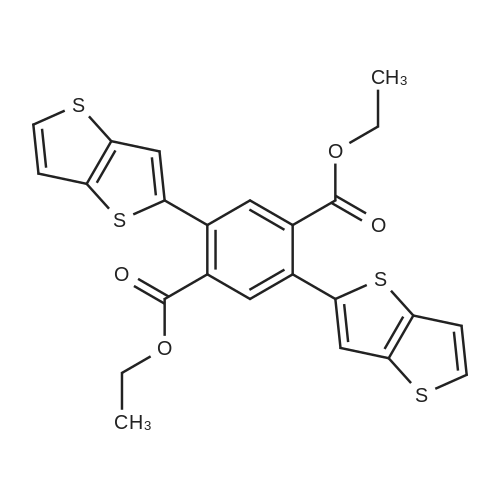
A 250 mL round bottom flask was charged with <strong>[18013-97-3]diethyl 2,5-dibromo-terephthalate</strong> (9.12 g, 24 mmol), 2-tri-n-butyltin-thieno[3,2-b]thiophene (25.76 g, 60 mmol) and tetratriphenylphosphine palladium (277.3 Mg, 0.24mmol). Nitrogen gas was purged three times, and toluene (120 mL) except oxygen was added, and the mixture was refluxed under nitrogen for 24 hours. After completion of the reaction, the mixture was cooled to room temperature, water was added, and dichloromethane was evaporated. The mixture was separated by silica gel column chromatography using dichloromethane as eluent to give diethyl 2,5-bis(thieno[3,2-b]thiophen-2-yl)terephthalate (8.98 g, yield 75%). |
| 75% |
With tetrakis(triphenylphosphine) palladium(0); In N,N-dimethyl-formamide; for 4h;Inert atmosphere; Reflux; |
Compound 5-2 (5 g, 13.2 mmol) in a 250 mL reaction flask under a nitrogen atmosphere,Add compound 1-2 (14.1 g, 32.9 mmol) and add N, N-dimethylformamide (50 mL) and stir.Pd (PPh3)4 (0.8 g, 0.7 mmol) was added to the reaction and stirred at reflux for 4 hours.The reaction is stirred while slowly cooling to room temperature (25 C.) (crystallization).The precipitated solid is filtered off and the filtered solid is washed with MeOH (30 mL).The filtered solid was dissolved in dichloromethane (50 mL), filtered using silica gel-pad, and the filtrate was concentrated under reduced pressure.The concentrate is recrystallized from Dichloromethane / MeOH (for 1 hour).The product solid is filtered off and the filtered solid is washed with MeOH (30 mL).The washed solid was dried overnight in a vacuum oven (temp. = 100 C.) to afford intermediate compound 5-1 (4.9 g, 75%). |
| 19.40 g |
With bis-triphenylphosphine-palladium(II) chloride; In tetrahydrofuran; N,N-dimethyl-formamide; for 20.5h;Reflux; |
To the solution of thieno[3,2-b]thiophene (17.529 g; 125.00 mmol) in THF anhydrous ( 50 cm3) was added at -78 C n-BuLi (50.0 cm3; 25.00 mmol) over 20 minutes. The mixture was stirred with cooling for 1 hour to yield a milky white suspension. The flask was lifted out of the cooling bath and was stirred without cooling for 30 min then cooled back to -78 C again. Tributyltin chloride (35.3 cm3; 125.00 mmol) was syringed into the solution in one portion and the mixture was stirred with the cooling bath for 16 hours then at 22C for 1 hour to yield a white suspension. The solid of <strong>[18013-97-3]diethyl 2,5-dibromo-terephthalate</strong> (19.00 g; 50.00 mmol), Pd(PPh3)2CI2 (1.0 g; 1.42 mmol; 2.84 mol%) and DMF anhydrous (50.0 cm3) were added sequentially and the mixture was heated to boiling for 0.5 hour. A distillation head was installed on the flask and 100 cm3 of the solvents were removed by distillation. The residue was then stirred at reflux for an additional 20 hours. The mixture was evaporated under vacuum to remove the low boiling solvents until a solid started crashing out. Methanol (200 cm3) was added to the residue and the precipitate was suction filtered off to yield a green-yellow crystalline solid. The solid was dissolved in hot chloroform (250 cm3) then filtered through a short silica plug (15 cm) which was washed with chloroform. The filtrate was concentrated to almost dryness and the solid was triturated with methanol followed by a suction filtration to yield the product as bright yellow crystals (19.40 g, 78%). 1H- NMR (CDC , 300 MHz): delta= 1.13 (t, J = 7.2 Hz, 3H), 4.25 (q, J = 7.2 Hz, 2H), 7.28 (dd, J1 = 5.2 Hz, J2 = 0.6 Hz, 1H), 7.30 (d, J = 0.6 Hz, 1 H), 7.40 (d, J = 5.2 Hz, 1 H), 7.89 (s, 1H). 13C-NMR (CDCI3, 75 MHz): delta= 13.8, 61.8, 119.3, 119.4, 127.4, 132.0, 133.8, 134.1 , 139.3, 139.9, 142.0, 167.4. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping