| 84.6% |
|
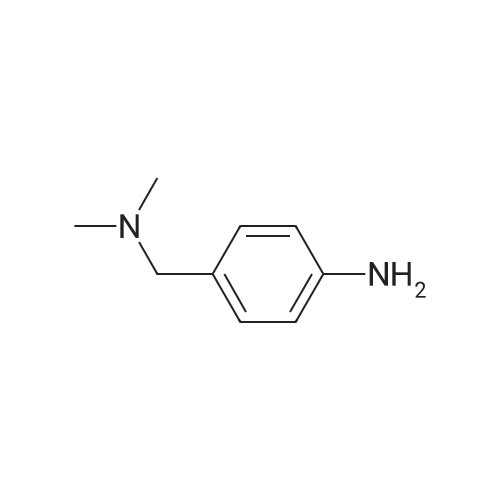
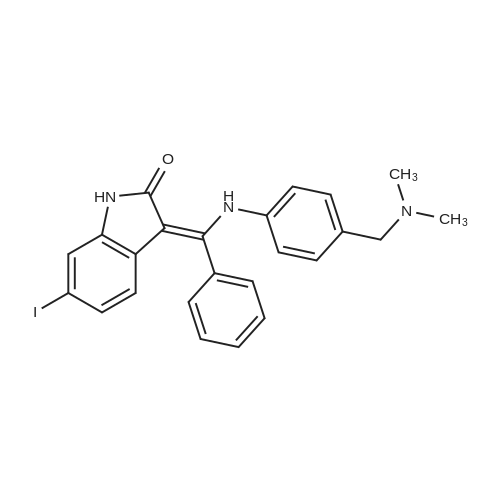
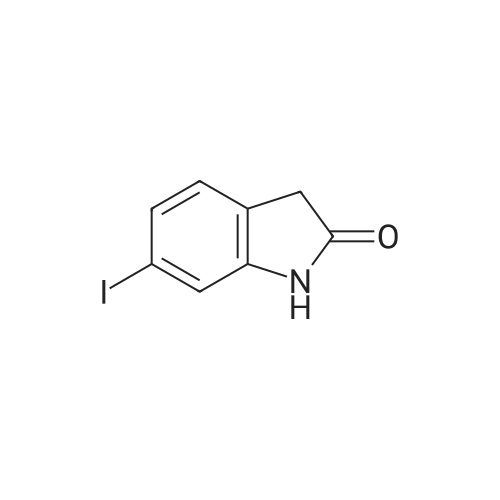
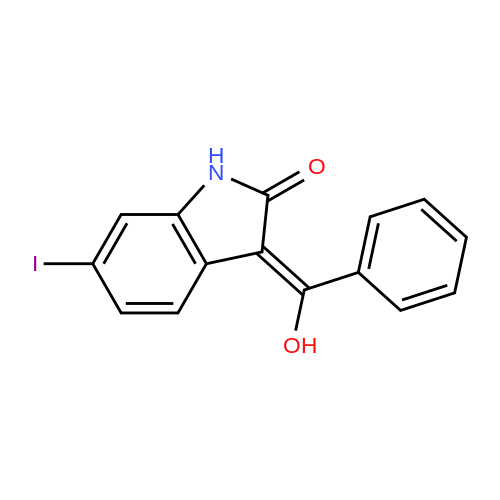
A nitrogen purged vessel is loaded with starting material 6-lodoindolinone (1 05kg,405mo1, 1,0 eq), catalyst 4-dimethylaminopyridine (DMAP) (2,52 kg) under argoncounter flow. Then triethylamine (145 kg, 3,5 eq) and solvent 2-methyltetrahydrofuran(605 kg) are charged to the vessel and the resulting solution is cooled to -15 00 to-S00 (preferentially -10C). Benzoylchloride (176,6 kg, 3,1 eq) is added to this mixture atan internal temperature of 100C to 50C within at least 30mm.The addition funnel is then flushed with 2-methyltetrahydrofuran (22 kg) and the reaction mixture is stirred for an additional hour at an internal temperature of 10 to 30C. If the content of starting material <strong>[919103-45-0]6-iodoindolinone</strong> is greater than 2,5 area%(H PLC), another portion of benzoylchloride (5,7 kg) is added to complete the reaction. If the content of starting material <strong>[919103-45-0]6-iodoindolinone</strong> is smaller than 2,5 area% (H PLC), lithium hydroxide (59,4 kg, 6,0 eq) is added in 5 differently sized portions (1st: 18,0 kg, 2?: 6,0 kg, 3: 6,0 kg, 41h: 15,0 kg, 5th: 14,4 kg) in a temperature controlled manner:After the two first portions, the mixture is stirred for 1 hour. After portion 3 and 4, the mixture is stirred for 30mm. After the last portion, the mixture is stirred for two hours. The reaction mixture (suspension) is then stirred for at least 12 hours at an internal temperature of 20 to 30 C. If the content of the non isolated intermediate of formula (V) is smaller than 0,5 area% (H PLC), water (525 L) is added and the mixture is heatedto an internal temperature of 60 to 7000 under stirring. Then the stirrer is switched off, the mixture is settled down and the phases are separated at an internal temperature of 60 to 70C. To the upper organic layer, water (525 L) is added and a second phase separation is carried out at an internal temperature of 60 to 70C. (Optionally, the mixture might be left stand at room temperature for up to 24 hours.) Then a partial solvent switch to tetrahydrofuran is carried out: Solvent is distilled off three times at a jacket temperature of 70C down to a residual volume of 390L followed by addition of tetrahydrofuran (1st: 233 kg, 2nd. 233 kg, 3rd. 117 kg). For crystallization, firstly, methanol (83 kg) is added.Optionally, the mixture might be left stand at room temperature for up to 24 hours. Secondly, water (112 L) is added at an internal temperature of 60 to 70C, followed by addition of conc. hydrochloric acid (156,2 kg). The addition funnel is flushed with water (20 L). The resulting suspension is cooled to 20 to 30C within at least 70mm (optionally, the mixture might be left stand at room temperature for up to 72 hours) and then to an internal temperature of minus 5 to 5 C within at least 30mm. The suspension is then centrifuged and the solid is washed with water (368 L) followed by methanol (112 kg) and dried at a jacket temperature of 50C until <= 1% of residual solvent is reached. The enol product of formula (IV) is obtained as solid in 84,6% yield. |
| 84.6% |
|
[0496] A nitrogen purged vessel is loaded with starting material 6-Iodoindolinone (105 kg, 405 mol, 1.0 eq), catalyst 4-dimethylaminopyridine (DMAP) (2.52 kg) under argon counter flow. Then triethylamine (145 kg, 3.5 eq) and solvent 2-methyltetrahydrofuran (605 kg) are charged to the vessel and the resulting solution is cooled to -15 C. to -5 C. (preferentially -10 C.). Benzoylchloride (176.6 kg, 3.1 eq) is added to this mixture at an internal temperature of -10 C. to 50 C. within at least 30 min. [0497] The addition funnel is then flushed with 2-methyltetrahydrofuran (22 kg) and the reaction mixture is stirred for an additional hour at an internal temperature of 10 to 30 C. If the content of starting material <strong>[919103-45-0]6-iodoindolinone</strong> is greater than 2.5 area % (HPLC), another portion of benzoylchloride (5.7 kg) is added to complete the reaction. If the content of starting material <strong>[919103-45-0]6-iodoindolinone</strong> is smaller than 2.5 area % (HPLC), lithium hydroxide (59.4 kg, 6.0 eq) is added in 5 differently sized portions (1st: 18.0 kg, 2nd: 6.0 kg, 3rd: 6.0 kg, 4th: 15.0 kg, 5th: 14.4 kg) in a temperature controlled manner: After the two first portions, the mixture is stirred for 1 hour. After portion 3 and 4, the mixture is stirred for 30 min. After the last portion, the mixture is stirred for two hours. The reaction mixture (suspension) is then stirred for at least 12 hours at an internal temperature of 20 to 30 C. If the content of the non isolated intermediate of formula (V) is smaller than 0.5 area % (HPLC), water (525 L) is added and the mixture is heated to an internal temperature of 60 to 70 C. under stirring. Then the stirrer is switched off, the mixture is settled down and the phases are separated at an internal temperature of 60 to 70 C. To the upper organic layer, water (525 L) is added and a second phase separation is carried out at an internal temperature of 60 to 70 C. (Optionally, the mixture might be left stand at room temperature for up to 24 hours.) Then a partial solvent switch to tetrahydrofuran is carried out: Solvent is distilled off three times at a jacket temperature of 70 C. down to a residual volume of 390 L followed by addition of tetrahydrofuran (1st: 233 kg, 2nd: 233 kg, 3rd: 117 kg). For crystallization, firstly, methanol (83 kg) is added. [0498] Optionally, the mixture might be left stand at room temperature for up to 24 hours. Secondly, water (112 L) is added at an internal temperature of 60 to 70 C., followed by addition of conc. hydrochloric acid (156.2 kg). The addition funnel is flushed with water (20 L). The resulting suspension is cooled to 20 to 30 C. within at least 70 min (optionally, the mixture might be left stand at room temperature for up to 72 hours) and then to an internal temperature of minus 5 to 5 C. within at least 30 min. The suspension is then centrifuged and the solid is washed with water (368 L) followed by methanol (112 kg) and dried at a jacket temperature of 50 C. until <=1% of residual solvent is reached. The enol product of formula (IV) is obtained as solid in 84.6% yield. |
|
|
0.88 g (7.23 mmol) 4-/V,/V-dimethylaminopyridine and 44.6 mL (101 .2 mmol) triethylamine are added successively to a suspension of 25.00 g (96.51 mmol) 6-iodo-1 ,3-dihydro-indol-2- one in 125.0 mL /V,/V-dimethylformamide. 27.81 g (197.8 mmol) benzoylchloride is added slowly at -10C to the reaction mixture and stirred for 2 h at -10C. After complete conversion (HPLC, Method A) 48.0 mL 10 M sodium hydroxide solution is added and stirred 1 h at room temperature. Then 350 mL water, 150 mL toluene and 80 mL cone, hydrochloric acid are successively added. The resulting precipitate is filtered, washed with water and toluene and dried at 50C in vacuo. [M+H]+: 364 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping