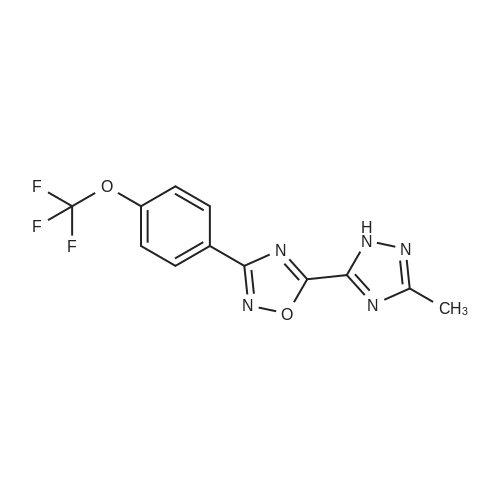
| 39% |
With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; ruphos; In toluene; at 140℃; for 18h;Inert atmosphere; |
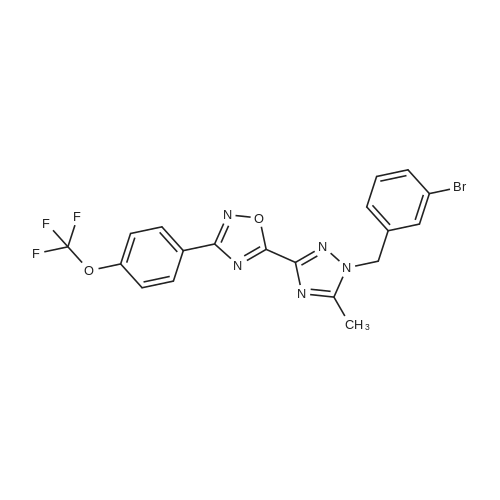
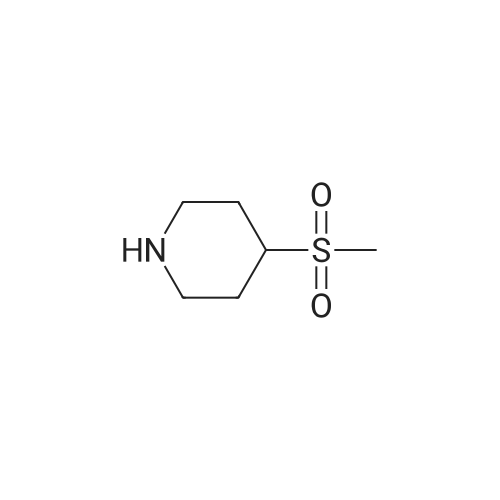
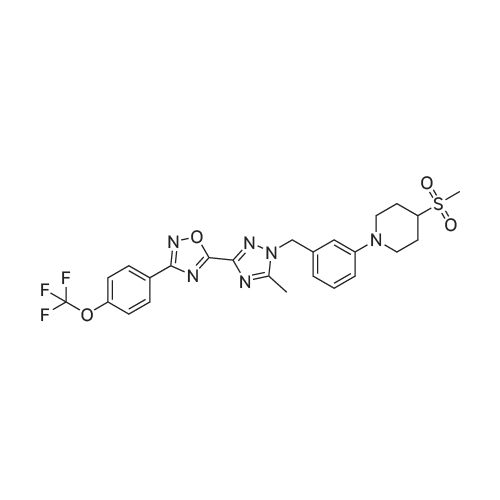
2-dicyclohexylphosphino-2?,6?-di-i-propoxy-1,1?-biphenyl (580 mg, 1.25 mmol) and tris(dibenzylideneacetone)dipalladium (760 mg, 0.83 mmol) were added to a mixture of 5-(1-(3-bromobenzyl)-5-methyl-1H-1,2,4-triazol-3-yl)-3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole (2.00 g, 4.16 mmol), <strong>[290328-55-1]4-(methylsulfonyl)piperidine</strong> (1.02 mg, 6.24 mmol), and t-BuONa (800 mg, 8.33 mmol) in toluene (80 ml), and the reaction mixture was degassed with argon for 3 min and then was heated to 140 C for 18 h under an argon atmosphere. The mixture was then cooled to RT, diluted with EtOAc (100 ml), filtered through a pad of celite, washed with EtOAc (100 ml), and concentrated under reduced pressure. The residue was purified using a silica gel chromatography column (PE:EtOAc = 1:1 with pureEtOAc) to produce a crude product, which was treated with EtOAc and Et2O (vol/vol = 1:9, 30 ml). The resulting suspension was stirred at RT for 30 min and thenfiltered to produce 5-(5-methyl-1-(3-(4-(methylsulfonyl)piperidin-1-yl)benzyl)-1H-1,2,4-triazol-3-yl)-3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole as a whitesolid (905 mg, 39%). 1H NMR (600 MHz, DMSO-d6): delta 8.22 (d, J = 8.8 Hz, 2 H),7.61 (d, J = 8.2 Hz, 2 H), 7.21 (t, J = 7.9 Hz, 1 H), 6.97 (bs, 1 H), 6.94 (dd, J = 8.3,2.4 Hz, 1 H), 6.64 (d, J = 7.5 Hz, 1 H), 5.48 (s, 2 H), 3.86 (bd, J = 13.4 Hz, 2 H), 3.28(m, 1 H), 2.94 (s, 3 H), 2.76 (m, 2 H), 2.57 (s, 3 H), 2.06 (bd, J = 13.4 Hz, 2 H), 1.68(ddd, J = 16.5, 12.5, 4.1 Hz, 2 H). 13C NMR (126 MHz, DMSO-d6): delta 169.0, 167.2,155.1, 150.7, 150.5, 147.9, 136.0, 129.6, 129.4, 124.9, 121.7, 119.9 (q, J = 258 Hz),117.8, 115.5, 115.2, 58.6, 52.3, 47.2, 37.4, 23.7, 11.7. 19F NMR (471 MHz,DMSO-d6): delta -56.6. HRMS (ESI+) m/z: [M + H]+ calculated for C25H26F3N6O4S,563.1683; found, 563.1675. |
|
With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; XPhos; In toluene; at 140℃; for 18h;Inert atmosphere; |
A mixture of 5-(l-(3-bromobenzyl)-5-methyl-lH-l,2,4-triazol-3-yl)-3- (4-(trifluoromethoxy)phenyl)-l,2,4-oxadiazole (Example 11, Step 1; 165 mg, 0.34 mmol), <strong>[290328-55-1]4-(methylsulfonyl)piperidine</strong> (62 mg, 0.38 mmol), and CS2CO3 (224 mg, 0.69 mmol) in toluene (2 mL) was degassed with argon for 5 min. Pd2(dba)3 (0.15 mg, 0.017 mmol) and dicyclohexyl(2',4',6'-triisopropyl-[l,l'-biphenyl]-2- yl)phosphine (33 mg, 069 mmol) were added and the reaction mixture was degassed a second time with argon for 5 min, then heated to 140 C for 18 h. The mixture was then cooled to RT, diluted with EtOAc (15 mL), filtered through a pad of Celite, and concentrated under reduced pressure. The crude product was purified by prep-HPLC (Mobile phase: A = 0.1% TFA/H20, B = 0.1%TFA/MeCN; Gradient: B = 40% - 80% in 12 min; Column: CI 8) to give the title compound as a white solid; MS (ES+) C25H25F3N604S requires: 562, found: 563 [M+H]+; *H NMR (600 MHz, DMSO-d6) delta 8.22 (d, J = 8.8 Hz, 2H), 7.61 (d, J = 8.2 Hz, 2H), 7.21 (t, J = 7.9 Hz, 1H), 6.97 (bs, 1H), 6.94 (dd, / = 8.3, 2.4 Hz, 1H), 6.64 (d, / = 7.5 Hz, 1H), 5.48 (s, 2H), 3.86 (bd, / = 13.4 Hz, 2H), 3.28 (m, 1H), 2.94 (s, 3H), 2.76 (m, 2H), 2.57 (s, 3H), 2.06 (bd, J = 13.4 Hz, 2H), 1.68 (ddd, / = 16.5, 12.5, 4.1 Hz, 2H); 19F NMR (282 MHz, DMSO-d6) delta -56.6. |
|
|
Step 4 4-Methanesulfonyl-1-{3-[(5-methyl-3-{3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl}-1H-1,2,4-triazol-1-yl)methyl]phenyl}piperidine A mixture of 5-(1-(3-bromobenzyl)-5-methyl-1H-1,2,4-triazol-3-yl)-3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole (165 mg, 0.34 mmol), <strong>[290328-55-1]4-(methylsulfonyl)piperidine</strong> (62 mg, 0.38 mmol), and Cs2CO3 (224 mg, 0.69 mmol) in toluene (2 mL) was degassed with argon for 5 min. Pd2(dba)3 (0.15 mg, 0.017 mmol) and dicyclohexyl(2',4',6'-triisopropyl-[1,1'-biphenyl]-2-yl)phosphine (33 mg, 069 mmol) were added and the reaction mixture was degassed a second time with argon for 5 min, then heated to 140 C. for 18 h. The mixture was then cooled to RT, diluted with EtOAc (15 mL), filtered through a pad of Celite, and concentrated under reduced pressure. The crude product was purified by prep-HPLC (Mobile phase: A=0.1% TFA/H2O, B=0.1% TFA/MeCN; Gradient: B=40%-80% in 12 min; Column: C18) to give the title compound as a white solid; MS (ES+) C25H25F3N6O4S requires: 562. found: 563 [M+H]+; 1H NMR (600 MHz, DMSO-d6) delta 8.22 (d, J=8.8 Hz, 2H), 7.61 (d, J=8.2 Hz, 2H), 7.21 (t, J=7.9 Hz, 1H), 6.97 (bs, 1H), 6.94 (dd, J=8.3, 2.4 Hz, 1H), 6.64 (d, J=7.5 Hz, 1H), 5.48 (s, 2H), 3.86 (bd, J=13.4 Hz, 2H), 3.28 (m, 1H), 2.94 (s, 3H), 2.76 (m, 2H), 2.57 (s, 3H), 2.06 (bd, J=13.4 Hz, 2H), 1.68 (ddd, J=16.5, 12.5, 4.1 Hz, 2H); 19F NMR (282 MHz, DMSO-d6) delta -56.6. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping