Alternatived Products of [ 157891-84-4 ]
Product Details of [ 157891-84-4 ]
| CAS No. : | 157891-84-4 |
MDL No. : | MFCD29921812 |
| Formula : |
C20H22O3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
310.39
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 157891-84-4 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 157891-84-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 157891-84-4 ]
- 1
-
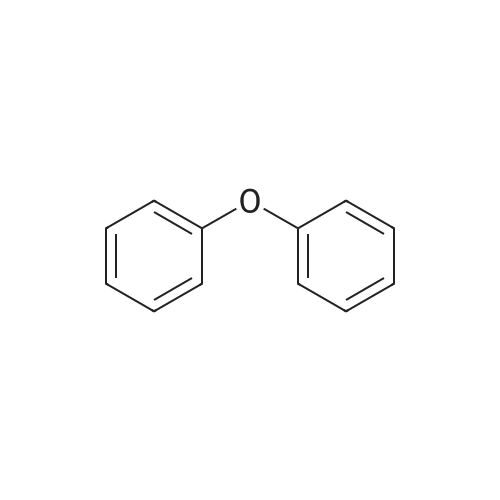 [ 101-84-8 ]
[ 101-84-8 ]

-
 [ 79-30-1 ]
[ 79-30-1 ]

-
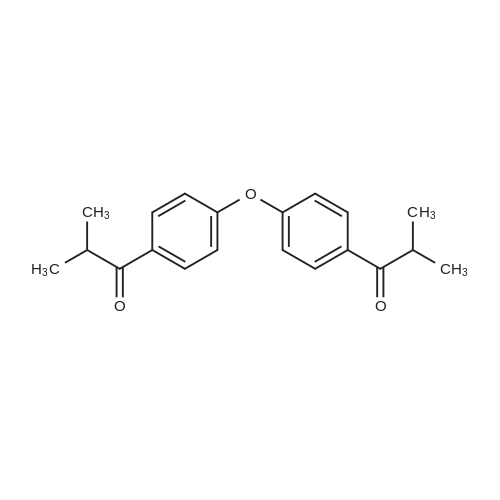 [ 157891-84-4 ]
[ 157891-84-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With Aluminum Chloride at 5 - 50℃; for 3h; |
3.a
15.33g of aluminum chloride 1 15mmoles) were added in portions in one hour to a solution of 8.6g of diphenylether (50mmoles) and 1 1 .97 g of isobutyryl chloride (1 l Ommoles) under stirring keeping the temperature between 5° and 150C. The mixture was maintained under stirring for an additional hour at 150C then heated at 5O0C for one hour. The mixture was treated with 100ml of water under stirring. The organic layer was separated obtaining 1 I g of yellow oil that was used for the next step without purification. A sample was crystallized from petroleum ether 40°-65°C affording a whitish solid with mp 540C.Hl NMR(300MHz, CDCb):δ: 7.98 (d, 4H); 7.04 (d, 4H); 3.45-3.55(m, 2H); 1 .21 (d, 12H). |
|
With Aluminum Chloride In water monomer at 5 - 50℃; for 2h; |
3.a
a) Acylation Synthesis of 1-[4-(4-isobutyryl-phenoxy)-phenyl]-2-methyl-propane-1-one. 15.33 g of aluminum chloride 115 mmoles) were added in portions in one hour to a solution of 8.6 g of diphenylether (50 mmoles) and 11.97 g of isobutyryl chloride (110 mmoles) under stirring keeping the temperature between 5° and 15° C. The mixture was maintained under stirring for an additional hour at 15° C. then heated at 50° C. for one hour. The mixture was treated with 100 ml of water under stirring. The organic layer was separated obtaining 11 g of yellow oil that was used for the next step without purification. A sample was crystallized from petroleum ether 40°-65° C. affording a whitish solid with mp 54° C. H1NMR(300 MHz, CDCl3):δ: 7.98 (d, 4H); 7.04 (d, 4H); 3.45-3.55(m, 2H); 1.21 (d, 12H). |
|
With Aluminum Chloride In dichloromethane at 0℃; |
|
|
With Aluminum Chloride In chlorobenzene at -5℃; for 0.5h; |
1.1-1.2; 3
(1) in the reaction unit, the diphenyl ether of 221.3g (1.3mol) is mixed with 300mL of chlorobenzene, and the temperature is cooled to -5°C, the reaction unit is connected to the tail gas absorption device, and the tail gas absorbent is ice water. Add 373.3g (2.8mol) of anhydrous aluminum chloride in the middle; then dropwise add 297.8g (2.8mol) of isobutyryl chloride, keep stirring for reaction, and TLC detects until the reaction is complete; (2) mixing the reaction solution obtained in step (1) with the ice water in the tail gas absorption device, stirring for 0.5h, leaving it to stand in a separatory funnel to separate the two phases, and washing the organic phase with water to obtain a compound solution of formula b; HPLC detects content > 95%; |

Reference:
[1]Current Patent Assignee: LAMBERTI S.P.A. - WO2009/135895, 2009, A1
Location in patent: Page/Page column 14
[2]Current Patent Assignee: LAMBERTI S.P.A. - US2011/65962, 2011, A1
Location in patent: Page/Page column 4
[3]Liang, Yu-Feng; Jiao, Ning
[Angewandte Chemie - International Edition, 2014, vol. 53, # 2, p. 548 - 552][Angew. Chem., 2014, vol. 53, # 2, p. 558 - 562,5]
[4]Current Patent Assignee: TIANJIN JIURI NEW MAT CO LTD - CN114349618, 2022, A
Location in patent: Paragraph 0035; 0071-0074; 0094-0095
- 2
-
 [ 157891-84-4 ]
[ 157891-84-4 ]

-
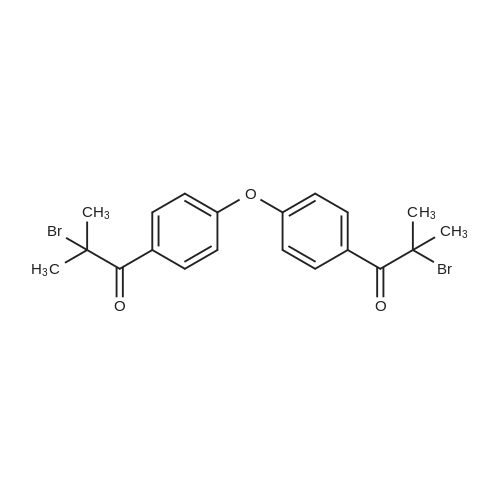 [ 157891-77-5 ]
[ 157891-77-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hypochlorite; hydrogen bromide In water at 45 - 60℃; for 0.666667h; |
3.b; G
13.73g of NaCIO 12% (23mmoles) were slowly added in 20' to a stirred suspension of 3.03g of of l-[4-(4-isobutyryl-phenoxy)-phenyl]-2-methyl-propane-l-one (9.8 mmoles) in 7.04g of hydrobromic acid 48% (41.2 mmoles) at 450C. Then the mixture was heated at 6O0C for 20'. The reaction was checked by TLC (SiCte, toluene). The whole mixture was used for the next step without purification, |
|
With hydrogen bromide; dihydrogen peroxide In water at 20 - 70℃; for 1.33333h; |
3.b; F
Synthesis of 2-bromo-1-{4-[4-(2-bromo-2-methyl-propionyl)-phenoxy]-phenyl}-2-methyl-propan-1-one. (Method F) 2.21 g of hydrogen peroxide 33% (21.5 mmoles) were added in 20' to a stirred suspension of 3.03 g of 1-[4-(4-isobutyryl-phenoxy)-phenyl]-2-methyl-propane-1-one (9.8 mmoles) in 7.04 g of hydrobromic acid 48% (41.8 mmoles) at 20° C. Then the mixture was heated at 70° C. for one hour. The reaction was checked by TLC (SiO2, toluene). The whole mixture was used for the next step without purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







