|
With potassium hydroxide In ethanol |
|
|
With potassium hydroxide In ethanol Heating; |
|
|
With potassium hydroxide In ethanol |
|
|
With potassium hydroxide In methanol Heating; |
|
|
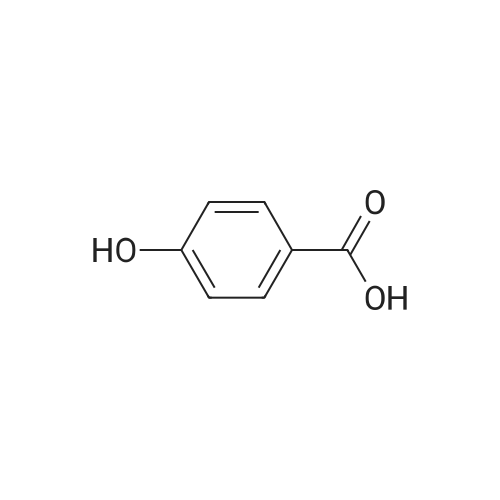
Stage #1: 4-hydroxy-benzoic acid With potassium hydroxide In methanol
Stage #2: hexadecanyl bromide In methanol Heating; Further stages.; |
|
|
With potassium hydroxide In methanol Reflux; |
|
|
With potassium hydroxide In ethanol |
|
|
With potassium hydroxide In ethanol Reflux; |
|
|
With potassium hydroxide In ethanol Reflux; |
|
|
Stage #1: 4-hydroxy-benzoic acid With potassium hydroxide In MeOH or EtOH
Stage #2: hexadecanyl bromide In MeOH or EtOH Reflux; |
|
|
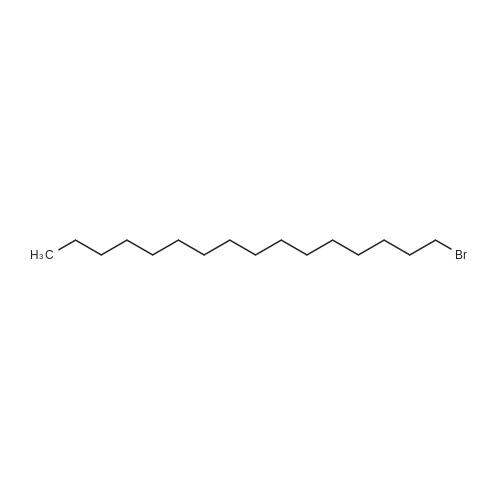
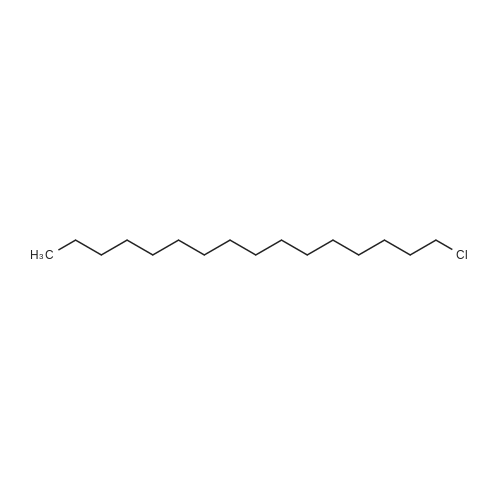
Stage #1: hexadecanyl bromide; 4-hydroxy-benzoic acid With potassium hydroxide at 70℃; Reflux;
Stage #2: Acidic aq. solution; Cooling with ice; |
|
|
Stage #1: 4-hydroxy-benzoic acid With potassium hydroxide
Stage #2: hexadecanyl bromide In ethanol Reflux; |
|
|
With potassium hydroxide In ethanol |
|
|
With potassium carbonate In methanol; ethanol Reflux; |
|
|
With potassium hydroxide In methanol |
|
|
With potassium hydroxide Reflux; |
|
|
Stage #1: hexadecanyl bromide; 4-hydroxy-benzoic acid With potassium hydroxide In ethanol for 14h; Reflux;
Stage #2: With hydrogenchloride In ethanol; lithium hydroxide monohydrate |
|
|
In methanol Reflux; |
|
|
With potassium hydroxide |
|
|
With potassium hydroxide In ethanol |
|
|
With potassium hydroxide |
|
|
With potassium hydroxide In ethanol for 7h; Reflux; |
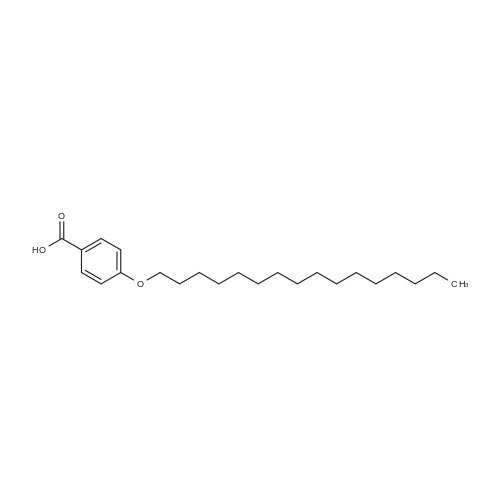
Synthesis of 4-decyloxybenzoic acid
General procedure: 4-Hydroxybenzoic acid (10 mmol, 1.381g) was dissolved in ethanol solution (25ml) of KOH (30 mmol, 1.68g). To this, 1-bromodecane (10 mmol, 2.07ml) was added drop by drop with constant stirring. The reaction mixture was refluxed for 7 h. The potassium salt thus obtained was hydrolysed with concentrate HCl (pH 2), which yielded a white precipitate. The precipitate was filtered off, washed with water and recrystallized from toluene. |
|
With potassium hydroxide In methanol |
|
|
With potassium hydroxide In methanol Reflux; |
|
|
With potassium hydroxide In ethanol for 7h; Reflux; |
|
|
With potassium hydroxide In methanol; ethanol Reflux; |
|
|
With potassium hydroxide In methanol Reflux; |
2.3.1. synthesis of 4-n-alkoxy benzoic acid (A)
General procedure: 4-n-alkoxy benzoic acid were synthesized by refluxing the mixture of 4-hydroxy benzoic acid(1 equiv.) with corresponding n-alkyl bromides (1 equiv.) in the presence of KOH (1.5 equiv.)and MeOH/ EtOH as a solvent [52]. |
|
With potassium hydroxide In methanol; ethanol Reflux; |
2.3.3. Synthesis of -n-alkoxy benzoic acid derivatives (C)
General procedure: 4-n-alkoxy benzoic acid were synthesized by refluxing the mixture of 4-hydroxy benzoic acid(1 equiv.) with corresponding n-alkyl bromides (1 equiv.) in the presence of KOH (1.5 equiv.)and MeOH/ EtOH as a solvent [38]. |
|
With potassium hydroxide In methanol Reflux; |
|
|
Stage #1: 4-hydroxy-benzoic acid With potassium hydroxide In methanol
Stage #2: hexadecanyl bromide In methanol at 64 - 66℃;
Stage #3: In lithium hydroxide monohydrate for 2h; Reflux; |
4.2.1. Synthesis of 4-n-alkyloxybenzoic acid (7b-m)
General procedure: To a solution of 4-hydroxy benzoic acid (3.5 g, 0.03 mmol, 1 eq)in methanol (25 mL) was added KOH (3.5 g, 0.06 mmol, 2.5 eq) andstirred for 8-10 min. To this solition, alkyl bromide (0.03 mmol,1.2 eq) was added and resulting mixture was refluxed at 64-66 C for 6-7 h. During this time, solid was separated out in reactionmixture. To this reaction mixture, 20% aq solution of KOH(5 mL) was added and refluxed for another 2 h to give clear solution.The reaction mixture was allowed to cool down to room temperatureand was acidified by 10% HCl solution (25 mL) followedby addition of ice-cold water to give solid. The solid separatedout was filtered, dried and recrystallized from absolute ethanolto give pure compound 7b-m. |
|
In ethanol Reflux; |
2.3.1. Synthesis of 4-n-alkoxy benzoic acid (A)
General procedure: 4-hydroxy benzoic acid alkylated by alkylating agent (R-Br), KOH, MeOH (C1 to C8)and Ethanol (C10 to C16), increasing reflux time period with increasing chain to yield corresponding 4-n-alkoxy benzoic acids (A), which was confirmed by IR and 1H NMR study [36]. |
|
With potassium hydroxide In ethanol |
2.2.4 4-n-Alkoxybenzoic acids [d]
General procedure: 4-n-alkoxybenzoic acids [D] were synthesized from 4-hydroxybenzoic acid by employing a Williamson’s ether synthesis protocol [79]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping