Alternatived Products of [ 16492-13-0 ]
Product Details of [ 16492-13-0 ]
| CAS No. : | 16492-13-0 |
MDL No. : | MFCD07776950 |
| Formula : |
C14H10ClNO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
243.69
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 16492-13-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 16492-13-0 ]
- 1
-
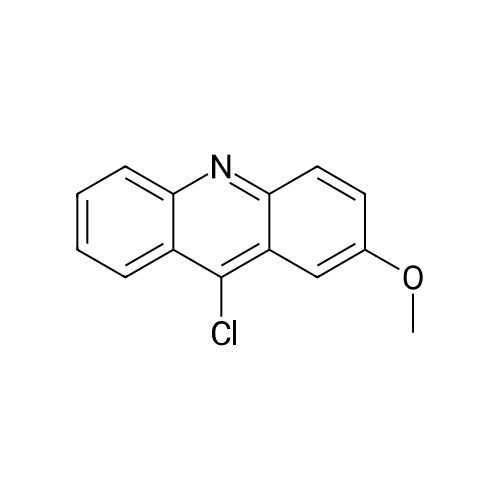 [ 16492-13-0 ]
[ 16492-13-0 ]

-
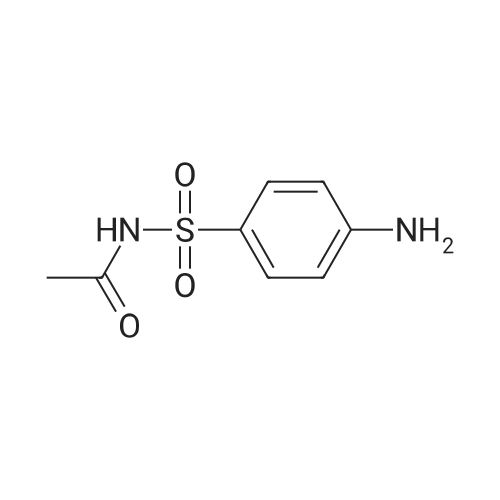 [ 144-80-9 ]
[ 144-80-9 ]

-
acetyl-[<i>N</i>-(2-methoxy-acridin-9-yl)-sulfanilyl]-amine
[ No CAS ]
- 2
-
 [ 16492-13-0 ]
[ 16492-13-0 ]

-
 [ 1950-68-1 ]
[ 1950-68-1 ]

-
C21H19N3O4S
[ No CAS ]
Reference:
[1]Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical,2005,vol. 44,p. 232 - 240
[2]Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical,2005,vol. 44,p. 232 - 240
- 3
-
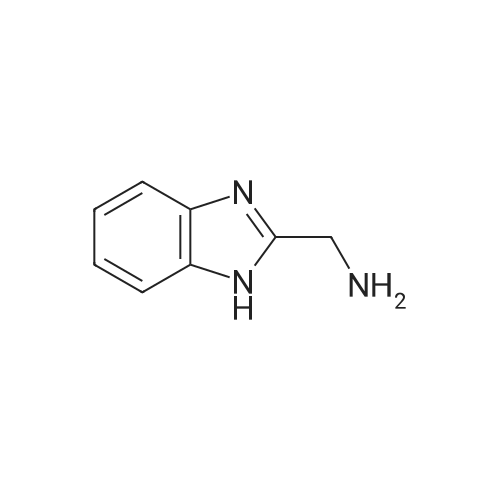 [ 5805-57-2 ]
[ 5805-57-2 ]

-
 [ 16492-13-0 ]
[ 16492-13-0 ]

-
N-((1H-benzo[d]imidazol-2-yl)methyl)-2-methoxyacridin-9-amine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 25% |
|
General procedure: 9-Chloroacridine or its derivatives (1.0 mmol) and phenol (10.0 mmol) were added into a 100 ml round-bottom flask and the mixture was stirred for 1 h at 60 C under argon atmosphere. Then benzimidazole derivatives (8a?8n, 8p?8q, 1.2 mmol) were added. The mixture was stirred under 120 °C for 2 h. Then the mixture was poured into a mixture of ethyl acetate (50 mL) and N-methyl morpholine (1 ml) to get the crude product as yellow precipitation. The crude product was recrystallized from ethylacetate. |
- 4
-
 [ 16492-13-0 ]
[ 16492-13-0 ]

-
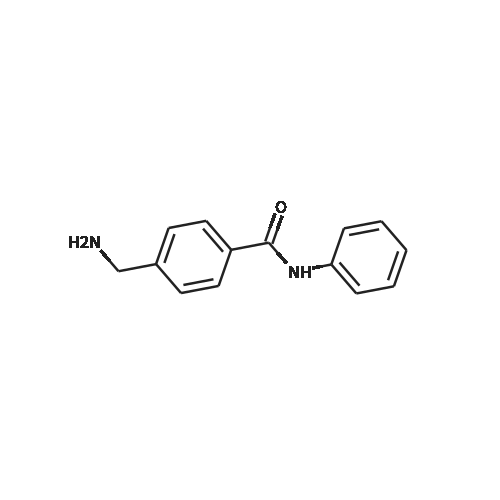 [ 22171-21-7 ]
[ 22171-21-7 ]

-
4-((2-methoxyacridin-9-amino)methyl)-N-phenylbenzamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In phenol at 90 - 100℃; Inert atmosphere; |
3.4. N-phenylbenzamide-4-methylamine acridine derivatives (9a-f)
General procedure: 9-chlorine acridine compounds 4a-4e (1.71 mmol) and 4-(aminomethyl)-N-phenylbenzamides 8a-b (2.23 mmol) dissolved in phenol (34.2 mmol), The apparatus was flushed with argon and the mixture was stirred at 90-100 °C until the TLC (dichloromethane/methanol=10/1, v/v) showed the disappearance of the starting material. The mixture was cooled to room temperature and ethyl acetate (20 mL) was added to the system, a lot of yellow precipitate was formed, then removed the supernatant, 20 mL ethyl acetate was added to the residue and stirred for 30 minutes at room temperature. After filtration, the solid was obtained and dissolved with dichloromethane (20 mL) and sodium hydroxide solution (20 mL). The organic layer was washed with water (20 mL×3) and saturated sodium chloride solution (20 mL), then dried over anhydrous magnesium sulfate, and evaporated in vacuum to yield crude product. Petroleum ether/ethyl acetate were used as the eluent in column chromatography to give pure product. |
Reference:
[1]Zhang, Bin; Dou, Zhende; Xiong, Zheng; Wang, Ning; He, Shan; Yan, Xiaojun; Jin, Haixiao
[Bioorganic and Medicinal Chemistry Letters, 2019, vol. 29, # 23]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








