Alternatived Products of [ 167484-19-7 ]
Product Details of [ 167484-19-7 ]
| CAS No. : | 167484-19-7 |
MDL No. : | MFCD18711456 |
| Formula : |
C14H18N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
230.31
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 167484-19-7 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 167484-19-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 167484-19-7 ]
- 1
-
 [ 121-44-8 ]
[ 121-44-8 ]

-
 [ 75-36-5 ]
[ 75-36-5 ]

-
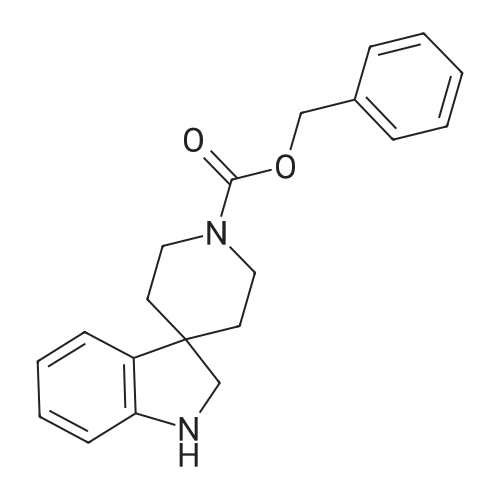 [ 167484-18-6 ]
[ 167484-18-6 ]

-
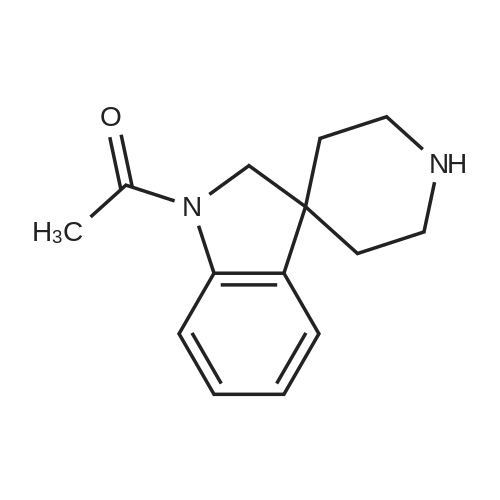 [ 167484-19-7 ]
[ 167484-19-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 2.93 g (77%) |
With acetic acid;palladium-carbon; In ethanol; dichloromethane; |
1-Acetyl-spiro(indoline-3,4'-piperidine) Acetyl chloride (1.4 mL, 19.9 mmol) was added to a solution of 5.35 g (16.6 mmol) of <strong>[167484-18-6]1'-benzyloxycarbonyl-spiro(indoline-3,4'-piperidine)</strong> in 33 mL of CH2 Cl2 and 3.2 mL (23.2 mmol) of Et3 N keeping the temperature between 0-5 C. by cooling in ice bath. After 10 min the cold bath was removed and reaction was stirred for 30 min at which time a TLC indicated complete reaction. The solution was diluted with CH2 Cl2 and washed with water, brine and dried over Na2 SO4. The filtrate was concentrated to a thick oil and the oil was dissolved in 40 mL of EtOH. Acetic acid (3 mL) and 0.8 g of 10% Pd/C were added to the solution and the resulting mixture was hydrogenated on a Parr apparatus for 3 h. The catalyst was filtered and washed with EtOAc and the combined filtrate was concentrated. The residue was partitioned between CH2 Cl2 and water and 2N NaOH was added to this mixture until the aqueous layer was basic. The layers were separated and the aqueous layer was extracted with CH2 Cl2. The combined organic layer was washed with brine, dried over Na2 SO4 and the filtrate was concentrated to give 2.93 g (77%) of the title compound sufficiently pure for use in the next reaction. |
| 2.93 g (77%) |
With acetic acid;palladium-carbon; In ethanol; dichloromethane; |
1-Acetyl-spiro(indoline-3,4'-piperidine) Acetyl chloride (1.4 mL, 19.9 mmol) was added to a solution of 5.35 g (16.6 mmol) of <strong>[167484-18-6]1'-benzyloxycarbonyl-spiro(indoline-3,4'-piperidine)</strong> in 33 mL of CH2 Cl2 and 3.2 mL (23.2 mmol) of Et3 N keeping the temperature between 0-5 C. by cooling in ice bath. After 10 min the cold bath was removed and reaction was stirred for 30 min at which time a TLC indicated complete reaction. The solution was diluted with CH2 Cl2 and washed with water, brine and dried over Na2 SO4. The filtrate was concentrated to a thick oil and the oil was dissolved in 40 mL of EtOH. Acetic acid (3 mL) and 0.8 g of 10% Pd/C were added to the solution and the resulting mixture was hydrogenated on a Parr apparatus for 3 h. The catalyst was filtered and washed with EtOAc and the combined filtrate was concentrated. The residue was partitioned between CH2 Cl2 and water and 2N NaOH was added to this mixture until the aqueous layer was basic. The layers were separated and the aqueous layer was extracted with CH2 Cl2. The combined organic layer was washed with brine, dried over Na2 SO4 and the filtrate was concentrated to give 2.93 g (77%) of the title compound sufficiently pure for use in the next reaction. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping





