Alternatived Products of [ 170570-78-2 ]
Product Details of [ 170570-78-2 ]
| CAS No. : | 170570-78-2 |
MDL No. : | MFCD03419786 |
| Formula : |
C11H8F2O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
242.18
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 170570-78-2 ]
Application In Synthesis of [ 170570-78-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 170570-78-2 ]
- 1
-
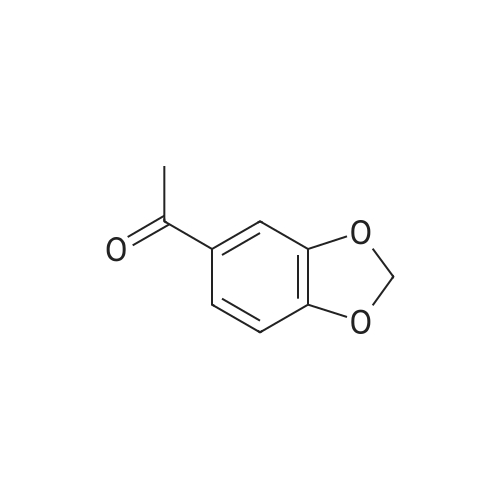 [ 3162-29-6 ]
[ 3162-29-6 ]

-
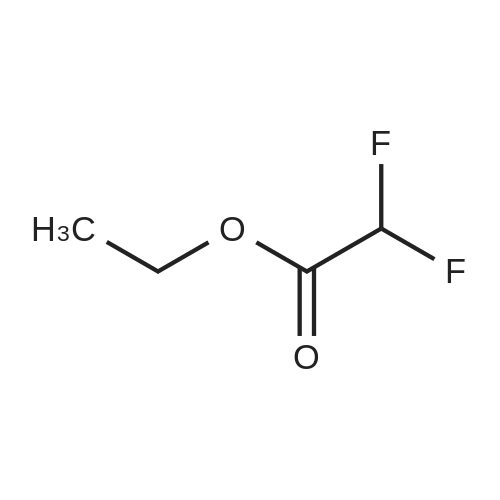 [ 454-31-9 ]
[ 454-31-9 ]

-
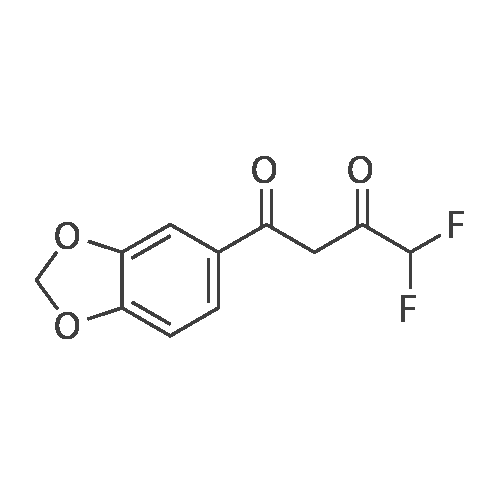 [ 170570-78-2 ]
[ 170570-78-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride; sodium methylate; |
Step 1. Preparation of 1-(1,3-benzodioxol-5-yl)-4,4-difluorobutane-1,3-dione Ethyl difluoroacetate (1.72 g, 11 mmol) was dissolved in ether (25 mL). To the stirred solution was added 25% sodium methoxide (2.38 g, 11 mmol) followed by 3',4'-(methylenedioxy)acetophenone (1.64 g, 10 mmol). After stirring 16 hours, 1N HCl (25 mL) was added. The organic layer was collected and washed with water (2*25 mL), dried over magnesium sulfate, filtered, and concentrated. The resulting crude dione was used in the next step without further purification or characterization. |
- 2
-
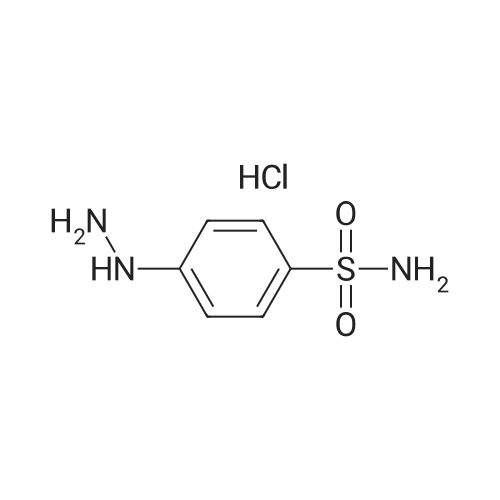 [ 17852-52-7 ]
[ 17852-52-7 ]

-
 [ 170570-78-2 ]
[ 170570-78-2 ]

-
4-[5-(1,3-benzodioxol-5-yl)-3-(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Step 1. Preparation of 1-(1,3-benzodioxol-5-yl)-4,4-difluorobutane-1,3-dione Ethyl difluoroacetate (1.72 g, 11 mmol) was dissolved in ether (25 mL). To the stirred solution was added 25% sodium methoxide (2.38 g, 11 mmol) followed by 3',4'-(methylenedioxy)acetoxyphenone (1.64 g, 10 mmol). After stirring 16 hours, 1N HCl (25 mL) was added. The organic layer was collected and washed with water (2*25 mL), dried over magnesium sulfate, filtered, and concentrated. The resulting crude dione was used in the next step without further purification or characterization. |
- 4
-
 [ 170570-78-2 ]
[ 170570-78-2 ]

-
5-(1,3-benzodioxol-5-yl)-4-[3-(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 84% |
In ethanol; water; |
Step 2. Preparation of 5-(1,3-benzodioxol-5-yl)-4-[3-(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide 1-(1,3-Benzodioxol-5-yl)-4,4-difluorobutane-1,3-dione from Step 1 (2.4 g, 10 mmol) was dissolved in ethanol (100 mL). To the stirred mixture was added 4-sulfonamidophenylhydrazine hydrochloride (2.46 g, 11 mmol) and heated to reflux for 16 hours. The mixture was cooled and water was added until crystals slowly appeared. Filtration yielded a light tan solid (3.3 g, 84%): mp 214-218 C.; 1 H NMR (D6 -DMSO): 7.86 (d, J=8.7 Hz, 2H), 7.51 (d, J=8.7 Hz, 2H), 7.49 (brs, 2H), 7.3-6.7 (m, 5H), 6.06(s, 2H). Anal. Calc'd for C17 H13 N3 SO4 F2: C, 51.91; H, 3.33; N, 10.68. Found: C, 51.90; H, 3.25; N, 10.65. |
| 84% |
In ethanol; water; |
Step 2. Preparation of 5-(1,3-benzodioxol-5-yl)-4-[3-(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide 1-(1,3-Benzodioxol-5-yl)-4,4-difluorobutane-1,3-dione from Step 1 (2.4 g, 10 mmol) was dissolved in ethanol (100 mL). To the stirred mixture was added 4-sulfonamidophenylhydrazine hydrochloride (2.46 g, 11 mmol) and heated to reflux for 16 hours. The mixture was cooled and water was added until crystals slowly appeared. Filtration yielded a light tan solid (3.3 g, 84%): mp 214-218 C. 1 H NMR (D6 -DMSO): 7.86 (d, J=8.7Hz, 2H), 7.51 (d, J=8.7Hz, 2H), 7.49 (brs, 2H), 7.3-6.7 (m, 5H), 6.06(s, 2H). Anal. Calc'd for C17 H13 N3 SO4 F2: C, 51.91; H, 3.33; N, 10.68. Found: C, 51.90; H, 3.25; N, 10.65. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







