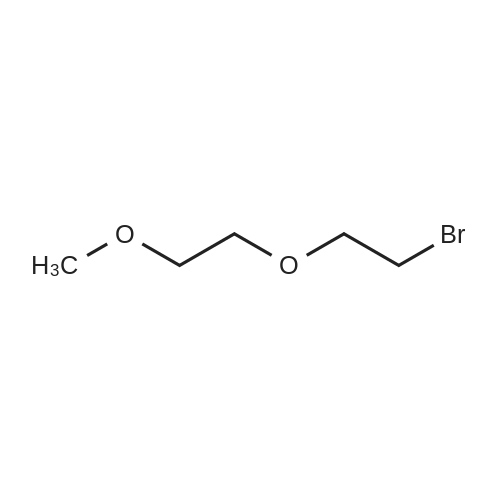
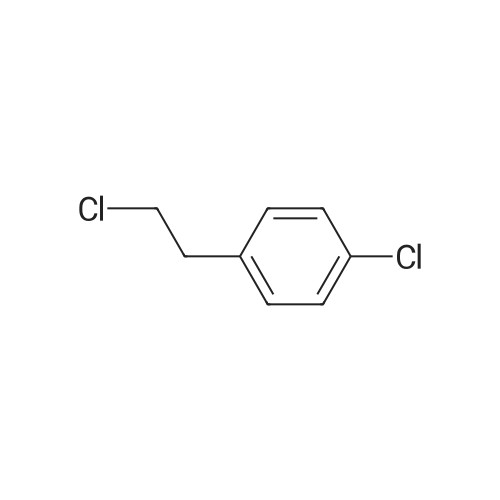
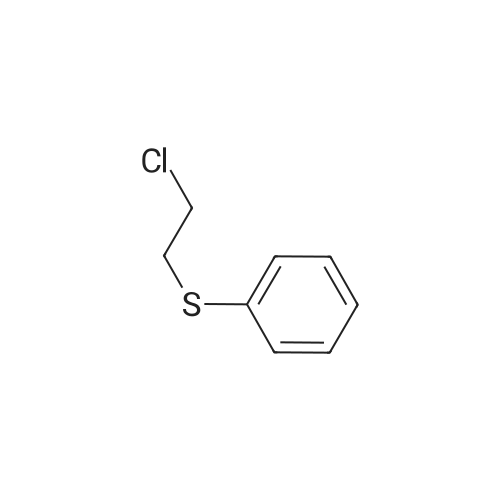
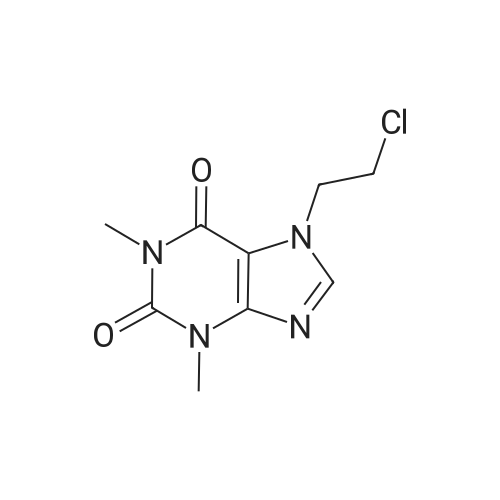
|
With hydrogen In ethanol at 20℃; |
2
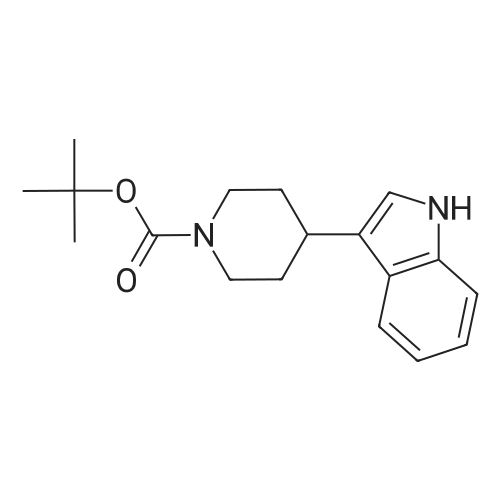
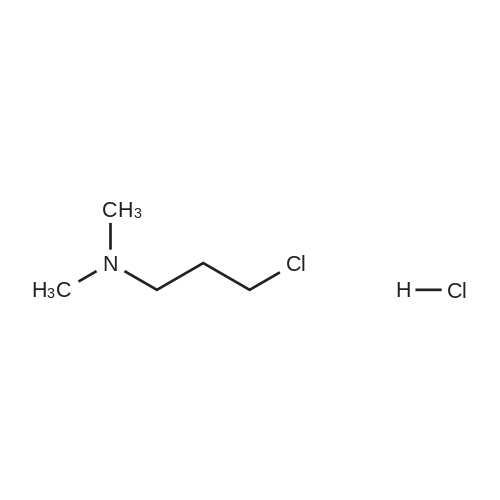
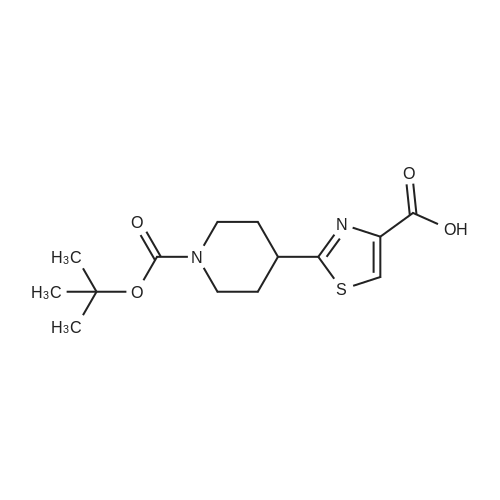
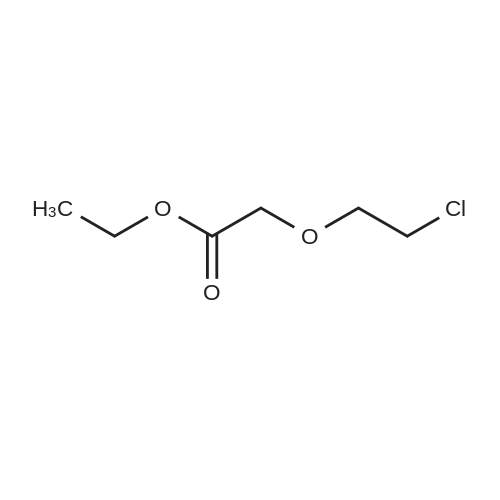
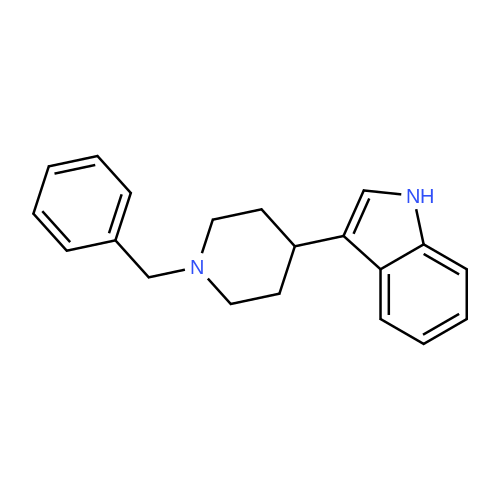
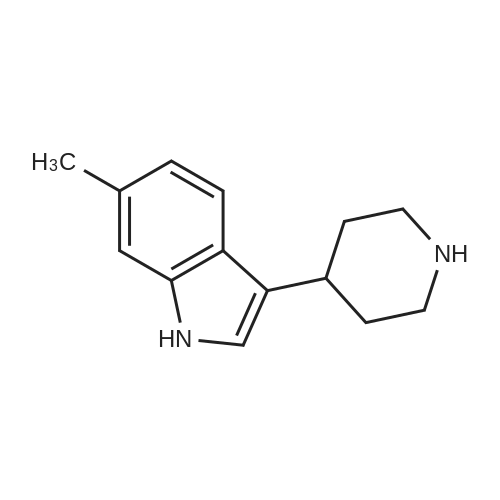
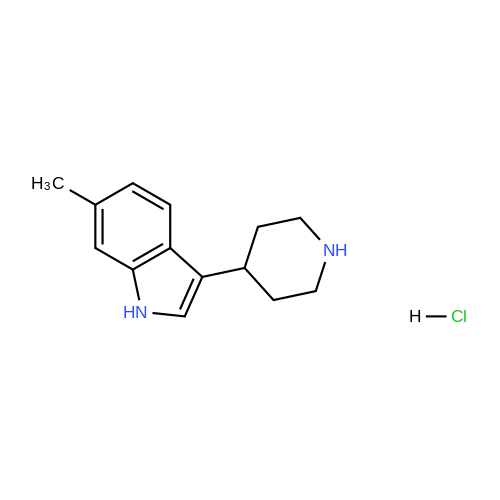
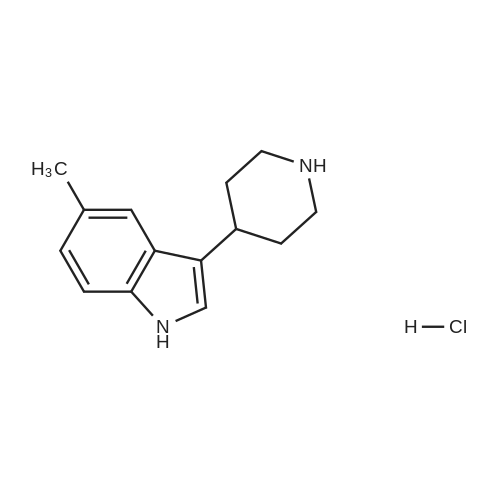
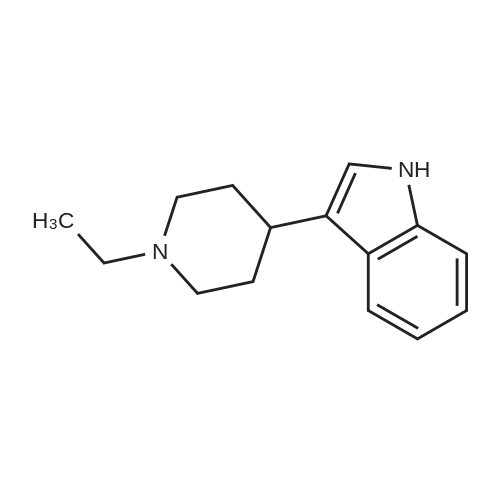
Example 2 ~ Synthesis and SAR of Diphenylbutylpiperidines Autophagy Inducers Based on Pimozide (2)Diphenylbutylpiperidines compounds, such as those described herein, can be prepared by SN2 substitution of halodiphenylbutyl compounds with piperidines (as described in Janneke, W.; Hulshof, H. F.; Vischer, H.P.; Verheij, S. A.; Fratantoni, Martine, J. S.; Iwan, J. P. Bioorg. Med. Chem. Lett. 2006, 14, 7213).As part of structure-activity relationship studies, modification of left-hand side of compound 2 were explored (Figures 13 and 14). Interestingly, compound 109 showed 2- fold increase in the activity of inducing autophagyModifications of the right-hand side of compound 2 were also explored. For example, synthetic variations on the P-1-56 fused ring scaffold are described in Figures 15 and 16. Reductive amination of N-Bocpiperidone with o-aminophenol was followed by cyclization with triphosgene and removal the Boc group. Substitution of the MsO group of piperidine 114 with compounds 113, 116 or 118 (6,5-fused heterocycles) followed by deprotection of Boc group by TFA afforded the desired compound 115, 117 or 119 (He, Y.; Yang, J., et al. Bioorg. Med. Chem. Lett. 2004, 14, 695). Reductive amination of N- Bocpiperidone 121 with o-aminophenol 120 followed by cyclization of 122 withtriphosgene and deprotection the Boc group yielded compound 123 (Flyren, K.; Bergquist, L. O.; et al. Bioorg. Med. Chem. Lett. 2007, 17, 3421). Treatment of piperidone 125 with indole gave compound 126, which was converted to compound 127 by hydrogenation. Reductive amination of 121 with indoline, prepared by reduction of indole, anddeprotection gave compound 130.Cyclization of compounds 120, 131 or 132 with 4-piperidinecarboxylic acid 133 give the desired compounds 134, 135 or 136 (He, Y.; Yang, J., etal. Bioorg. Med. Chem. Lett. 2004, 14, 695). Reductive amination of N-Boc piperidone with isoindoline prepared by reduction of phthalimide 137 and deprotection of the Boc group gave compound 138. Substitution of MsO group of piperidine 141 with phthalimide potassium salt followed by deprotection of Boc group afforded the desired compound 142. Treatment of 4-hydroxy piperidine 144 with 1,2,3-benzotriazole by Mitsunobu reaction followed by deprotection afforded two compounds 145 and 146 (Ruckle, T.; Biamonte, M., et al. J. Med. Chem. 2004, 47, 6921). Cyclization of compounds 121, 131 or 132 wuth 4-piperidine carboxylic acid gave compounds 134, 135 or 136 (respectively).Figures 18 and 19 showed the biological activity of selected P-l-56 compounds. As mentioned above, compound 109 showed 2-fold increased potent activity comparable to compound 2. A series of compounds with CN group have also been prepared; interestingly, some showed good results (147 vs 155, 151 vs 159, 153 vs 161) while some did not (149 vs 157, 150 vs 158, 152 vs 160). In the left series, compounds 149, 150, 152 and 153 showed 3 to 5-fold increased activity comparable to compound 2, while compounds 148, 151 and 154 showed no activity. In the right series, compounds 157, 158 and 160 showed 3 to 7-fold increased activity comparable to compound 2, while compounds 156 and 162 showed no activity. All of the above results indicate that the 4-position of piperidine ring can tolerate 6,5-fused heterocycle groups.The biological activity results of P-2-56 compounds is shown in Figure 20. These results suggested that compounds 163 and 166 are comparable to compound 2, while compounds 169 and 178 showed about a 4-fold increase in activity. However, compounds 164, 165, 170 and 177 showed no activity. Comparing the three isoelectronic compounds at the bottom of Figure 20, compound 166 showed the best result, while compounds 167 and 168 showed the same results. In contrast, compound 178 also showed the best result, while compounds 179 and 180 showed no activity.Encouraged by the biological activity results of DPBPs with 6,5-fused ring linked to the piperidine ring, compounds with 6,6-fused ring shown in Figures 21-24 were also prepared. Reductive amination of N-Boc piperidone with compound 182, prepared by reduction of isoquinoline 137, and deprotection of the Boc group gave compound 183. Cyclization of anthranilamide 184 with N-benzylpiperidone 185 followed by reduction of compound 186 afforded intermediate compound 187 (Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Kubo, K.; Shuto, K.; Kasuya, Y.; Shigenobl, K.; Hashikami, M.; Karashima, N. Chem. Pharm. Bull. 1985, 33, 1116). Cyclization 187 with CDI and deprotection of the Boc group gave compound 189. Cyclization 187 with different regents gave compounds 191, 193, 195 and 197 (Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Kubo, K.; Shuto, K.; Kasuya, Y.; Shigenobl, K.; Hashikami, M.; Karashima, N. Chem. Pharm. Bull. 1985, 33, 1116; and Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Kubo, K.; Shuto, K.; Hashimoto, T. Chem. Pharm. Bull. 1985, 33, 1104).The biological activity results of P-2-66 compounds is shown in Figures 23 and 24. Reductive amination of N-Boc piperidone with compound 199, prepared by reduction of quinoline 198, and deprotection of the Boc group gave compound 200. Treatment of salicylaldehyde 201 with compound 202, followed cyclization with CDI and deprotection of Bn group gave compound 205 (Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Kubo, K.; Shuto, K.; Hashimoto, T. Chem. Pharm. Bull. 1985, 33, 1104). Ring opening of compound 206 with 202 afforded intermediate 203 (Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Karasawa, A.; Kubo, K.; Shuto, K.; Kasuya, T. Chem. Pharm. Bull. 1986, 34, 1907). Cyclization 203 with different regents gave compounds 205, 207 and 209.Reductive amination of compound 210 with 211, followed by deprotection of the Boc group, gave compound 212. Cyclization 212 with different regents gave compounds 213- 217 (Takai, H.; Obase, H.; Nakamizo, N.; Teranishi, M.; Kubo, K.; Shuto, K.; Hashimoto, T. Chem. Pharm. Bull. 1985, 33, 1104).Compounds 234-237, which combined variations in both the left-hand side and right-hand side, we prepared and tested (Figure 28). Compounds 238 and 239 were also prepared and tested (Figure 29). Compound 239 (with a spiro structure) showed better results than Fluspirilene; this compound was easily prepared and has good solubility properties. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping