Alternatived Products of [ 179101-49-6 ]
Product Details of [ 179101-49-6 ]
| CAS No. : | 179101-49-6 |
MDL No. : | |
| Formula : |
C12H18N3O14P3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
521.20
|
Pubchem ID : | - |
| Synonyms : |
5-Propargylamino-dUTP
|
Chemical Name : | Ap-dutp |
Safety of [ 179101-49-6 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 179101-49-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 179101-49-6 ]
- 1
-
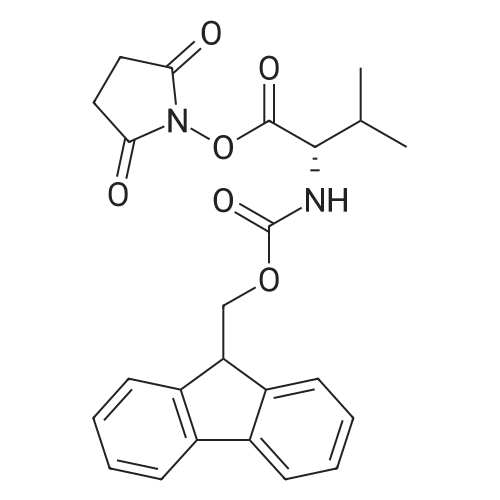 [ 130878-68-1 ]
[ 130878-68-1 ]

-
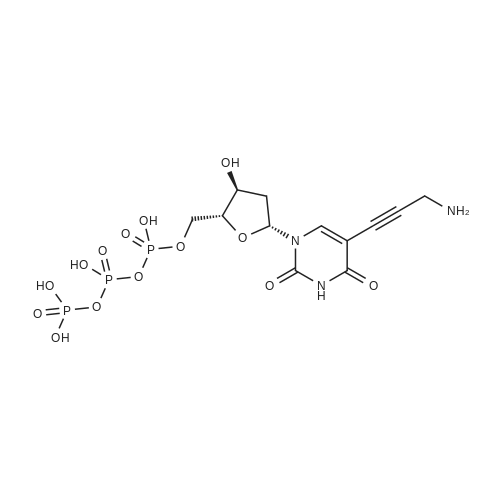 [ 179101-49-6 ]
[ 179101-49-6 ]

-
C32H37N4O17P3
[ No CAS ]
- 2
-
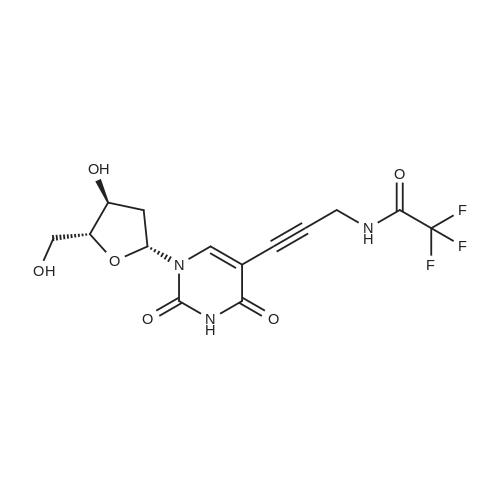 [ 115899-40-6 ]
[ 115899-40-6 ]

-
 [ 179101-49-6 ]
[ 179101-49-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
(0.16 mmol) of compound F3, 150 mg (0.32 mmol) of tri-n-butylamine pyrophosphate (E-4), 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one (E-3) (66 mg, 0.32 mmol) was placed in three reaction tubes. The tri-n-butylamine pyrophosphate was dissolved in 0.5 mL of anhydrous DMF and 0.6 mL of freshly distilled tri-n-butylamine was added and stirred for half an hour. A solution of 2-chloro-4H-1,3-benzodioxaphosphorin-4-one was dissolved in 0.5 mL of anhydrous DMF and the above tri-n-butylamine pyrophosphate solution was added by syringe under vigorous stirring half an hour. The mixture was then poured into F3 and stirred 1.5h. 5 mL of a 3% solution of iodine (9:1 Py/H2O) was added. After 15 min, 4 mL of water was added and the mixture was stirred for 2 h. Add 30mL anhydrous ethanol, frozen at night -20 C, centrifugation (3200r / min, 25 C) 20min. Dump the supernatant to precipitate and drain the solvent. The TEAB solution and concentrated aqueous ammonia were successively added and stirred at room temperature overnight. Evaporation of the solvent under reduced pressure gave a white solid which gave dUTP-NH2. The mobile phase: 20 mM TEAAc and CH3CH2OH, gradient wash, 0% to 20% CH3CH2OH (35 min); violet (violet); flow rate: 1 mL / min; column chromatography: External detector: 254 nm. At t=13.5 min, a product peak was formed. |
Reference:
[1]Journal of the American Chemical Society,2005,vol. 127,p. 15071 - 15082
[2]Journal of the American Chemical Society,1999,vol. 121,p. 9781 - 9789
[3]Bioorganic and Medicinal Chemistry,2014,vol. 22,p. 4384 - 4390
[4]Patent: CN104003902,2016,B .Location in patent: Paragraph 0094; 0095
- 3
-
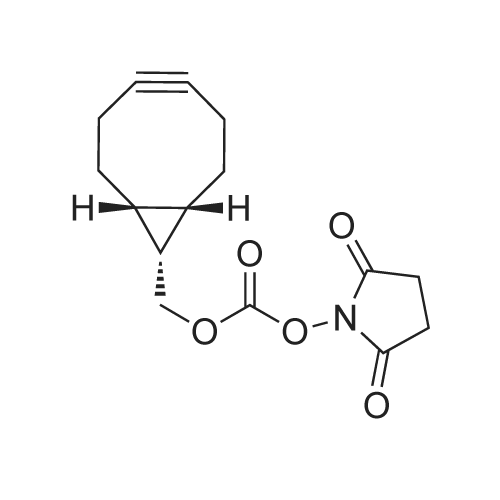 [ 1426827-79-3 ]
[ 1426827-79-3 ]

-
 [ 179101-49-6 ]
[ 179101-49-6 ]

-
C23H30N3O16P3
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water; N,N-dimethyl-formamide; at 55℃; for 4h; |
General procedure: The freeze-dried 5-aminopropargyl-2'-deoxyuridine-5'-triphosphate (20 O.D.) was dissolved in 40.0 μL water and 40.0 μL 1.0 M TEAB buffer (pH = 7.5). The active ester (2.0 mg) in DMF (80.0 μL) was added to the triphosphate solution. The mixture was kept at 55oC for 4 h and the solvent was then removed in vacuo. The products were purified by RP-HPLC (eluentA: 0.1 M TEAB buffer (pH = 7.5), B: 60 % acetonitrile in 0.1 M TEAB (pH = 7.5) buffer, 3.5% - 60 % buffer B in 20 min). The pure products were characterized by mass spectrometry. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






