| 97% |
With triethylamine In dichloromethane at 20℃; Cooling with ice; Inert atmosphere; |
25
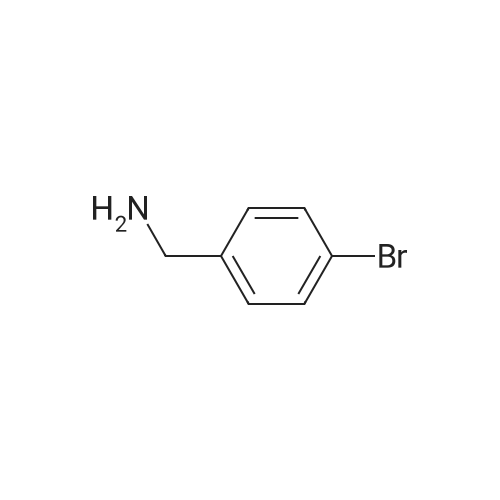
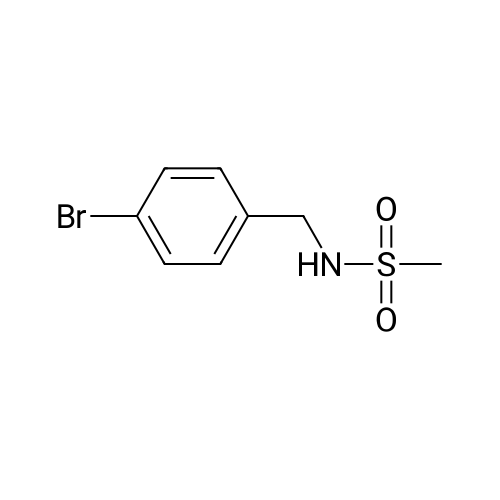
A solution of 4-bromobenzylamine (2.50 g, 13.5 mmol) and triethylamine (14.9 mmol, 1.5 g, 2.1 ml) in dichloromethane (50 ml) was cooled in an ice bath with stirring under argon. Methanesulfonyl chloride (1.55 g, 13.7 mmol, 1.05 ml) was added dropwise and the resulting mix was allowed to warm up to room temperature and stirred for 1 hour. The reaction mix was washed 3 times with water (20 ml) and the organic layer deied over sodium sulphate and reduced to minimum volume under reduced pressure to give the title compound as a colourless solid (3.45 g, 97%).1H-NMR (400 MHz, CDCl3) δ: 7.51 (2H, m), 7.25 (2H, m), 4.67 (1H, m), 4.29 (2H, d, J=6 Hz), 2.90 (3H, s); LC/MS Retention time 2.39 mins/(ES-) 262 & 264 (M-H, C8H10BrNO2S requires 263 & 265). |
| 95% |
With pyridine at 0℃; for 1h; |
4.1 Step 1
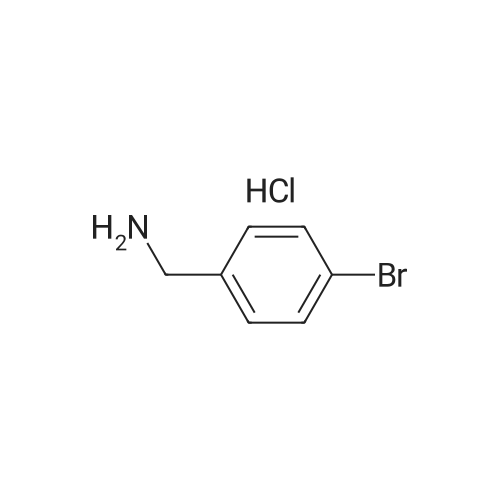
Step 1: To a stirred solution of No.78 (4-bromophenyl)methanamine (500 mg, 2.687 mmol) in No.64 pyridine were added No.47 methanesulfonyl chloride (0.4 mL, 5.106 mmol) at 0° C. The reaction mixture was stirred for 1 h, then diluted with dichloromethane. The mixture was washed with water. The organic layer was dried over magnesium sulfate and filtered. The filtrate removed in vacuo. The crude was purified by column chromatography. No.79 N-(4-bromobenzyl)methane-sulfonamide (675 mg) was obtained in 95% yield.[0419]Step 2: To a stirred solution of No.79 N-(4-bromobenzyl)methanesulfonamide (675 mg, 2.555 mmol) in No.56 dimethylformamide were added No.81 ethyl 2-chloropropionate (0.42 mL), No.82 manganese (280 mg) and No.83 (2,2′-bipyridine)nickel(II)-dibromide (67 mg, 0.17885 mmol). No.84 Trifluoroacetic acid (2 drops) was added. The reaction mixture was stirred for 36 h at 60° C. After cooling down to room temperature, the mixture was hydrolysed by 1N No.85 HCl and extracted with diethyl ether. The organic layer was dried over magnesium sulfate and filtered. The filtrate removed in vacuo. The crude was purified by column chromatography to obtain No.86 ethyl 2-(4-(methylsulfonamidomethyl)phenyl)propanoate (325 mg).[0420]Step 3: To a stirred solution of No.86 ethyl 2-(4-(methylsulfonamidomethyl)phenyl)propanoate (325 mg, 1.139 mmol) in co-solvent with No.27 tetrahydrofuran and No.37 water (1:1) were added No.88 sodium hydroxide (114 mg, 2.8475 mmol). The reaction mixture was refluxed for 16 h, then cooled to room temperature, acidified to pH 3-4 with acetic acid. The residue dissolved in No.43 ethyl acetate and washed with water and brine. The organic layer was dried over magnesium sulfate and filtered. The filtrate removed in vacuo. The crude was purified by column chromatography to give No.89 2-(4-(methylsulfonamidomethyl)phenyl)propanoic acid (74 mg, 25%).[0421]Step 4: To a stirred solution of No.89 2-(4-(methylsulfonamidomethyl)phenyl)propanoic acid (60 mg, 0.233 mmol) and No.36 (2-m-tolyl-6-(trifluoromethyl)pyridin-3-yl)methanamine (62 mg, 0.233 mmol) in No.71 acetonitrile were added No.33 N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (67 mg, 0.349 mmol), No.34 1-hydroxybenzotriazole (47 mg, 0.349 mmol) and No.35 triethylamine (0.08 mL, 0.582 mmol). The reaction mixture was stirred for 15 h at room temperature. The residue dissolved in No.43 ethyl acetate and washed with water and brine. The organic layer was dried over magnesium sulfate and filtered. The filtrate removed in vacuo. The crude was purified by column chromatography to obtain No.91 2-(4-(methylsulfonamidomethyl)phenyl)-N-((2-m-tolyl-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (example 4) (81 mg, 69%).[0422]1H NMR (300 MHz, CDCl3) 7.75 (d, 1H, J=8.07 Hz, Ar), 7.57 (d, 1H, J=8.07 Hz, Ar), 7.26 (m, 8H, Ar), 5.54 (t, 1H, NH), 4.63 (t, 1H, NH), 4.45 (d, 2H, CH2), 4.30 (d, 2H, CH2), 3.50 (q, 1H, CH), 2.91 (s, 3H, mesyl), 2.38 (s, 3H, methyl), 1.47 (d, 3H, methyl). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping