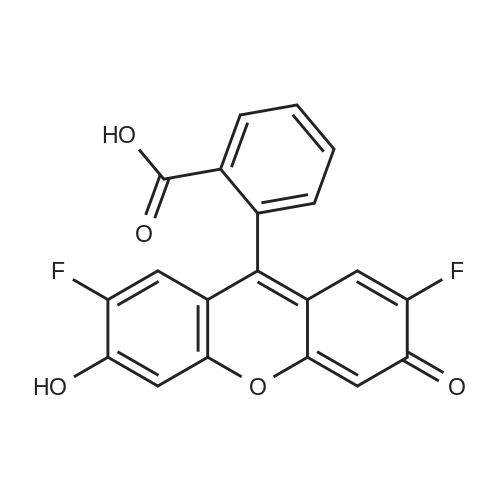
Alternatived Products of [ 195136-58-4 ]
Product Details of [ 195136-58-4 ]
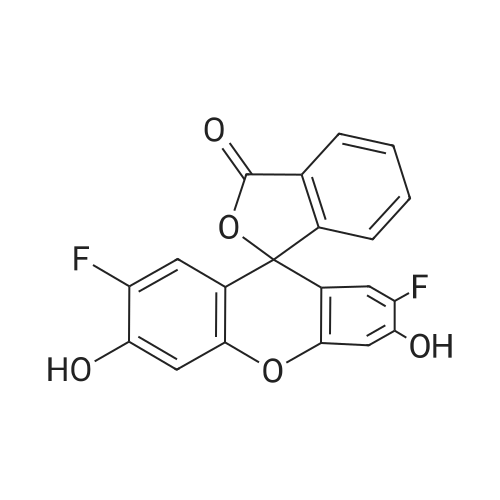
CAS No. : 195136-58-4
MDL No. : MFCD01311849
Formula :
C20 H10 F2 O5
Boiling Point : -
Linear Structure Formula : -
InChI Key : FJXJIUHGLVUXQP-UHFFFAOYSA-N
M.W :
368.29
Pubchem ID : 10292443
Synonyms :
Oregon Green™ 488;OG 488;2’,7’-Difluorofluorescein
Calculated chemistry of [ 195136-58-4 ]
Physicochemical Properties
Num. heavy atoms : 27
Num. arom. heavy atoms : 18
Fraction Csp3 : 0.05
Num. rotatable bonds : 0
Num. H-bond acceptors : 7.0
Num. H-bond donors : 2.0
Molar Refractivity : 88.66
TPSA : 75.99 Ų
Pharmacokinetics
GI absorption : High
BBB permeant : No
P-gp substrate : Yes
CYP1A2 inhibitor : Yes
CYP2C19 inhibitor : No
CYP2C9 inhibitor : Yes
CYP2D6 inhibitor : No
CYP3A4 inhibitor : Yes
Log Kp (skin permeation) : -5.98 cm/s
Lipophilicity
Log Po/w (iLOGP) : 2.59
Log Po/w (XLOGP3) : 3.62
Log Po/w (WLOGP) : 4.68
Log Po/w (MLOGP) : 3.24
Log Po/w (SILICOS-IT) : 4.19
Consensus Log Po/w : 3.66
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 0.0
Bioavailability Score : 0.55
Water Solubility
Log S (ESOL) : -4.9
Solubility : 0.00467 mg/ml ; 0.0000127 mol/l
Class : Moderately soluble
Log S (Ali) : -4.9
Solubility : 0.0046 mg/ml ; 0.0000125 mol/l
Class : Moderately soluble
Log S (SILICOS-IT) : -6.69
Solubility : 0.0000752 mg/ml ; 0.000000204 mol/l
Class : Poorly soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 0.0 alert
Leadlikeness : 2.0
Synthetic accessibility : 3.5
Safety of [ 195136-58-4 ]
Application In Synthesis of [ 195136-58-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 195136-58-4 ]
1
[ 1656-44-6 ]
[ 195136-58-4 ]
[ 827020-43-9 ]
Yield Reaction Conditions Operation in experiment
67%
With 2,6-dimethylpyridine In dichloromethane at 20℃;
Reference:
[1]Maeda, Hatsuo; Yamamoto, Kayoko; Nomura, Yoko; Kohno, Iho; Hafsi, Leila; Ueda, Noritsugu; Yoshida, Shoko; Fukuda, Masako; Fukuyasu, Yuka; Yamauchi, Yuji; Itoh, Norio
[Journal of the American Chemical Society, 2005, vol. 127, # 1, p. 68 - 69]
2
[ 195136-58-4 ]
[ 913689-08-4 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 67 percent / 2,6-lutidine / CH2 Cl2 / 20 °C
2: aq. hypoxanthine; xanthine oxidase / dimethylsulfoxide; various solvent(s) / 37 °C / pH 7.4
Reference:
[1]Maeda, Hatsuo; Yamamoto, Kayoko; Nomura, Yoko; Kohno, Iho; Hafsi, Leila; Ueda, Noritsugu; Yoshida, Shoko; Fukuda, Masako; Fukuyasu, Yuka; Yamauchi, Yuji; Itoh, Norio
[Journal of the American Chemical Society, 2005, vol. 127, # 1, p. 68 - 69]
Yield Reaction Conditions Operation in experiment
90 Preparation of 2',7'-difluorofluorescein, tetra-O-acetylgalactoside (90)
Example 90 Preparation of 2',7'-difluorofluorescein, tetra-O-acetylgalactoside (90) Using general method O, 2',7'-difluorofluorescein (29, 70 mg, 0.19 mmol, Rf =0.13 (CHCl3 /methanol/acetic acid 50:5:1)) gives Compound 90 as 0.12 g (92%) of a yellow foam: Rf =0.31 (CHCl3 /methanol/acetic acid 50:5:1).
4
[ 85-44-9 ]
[ 103068-41-3 ]
[ 195136-58-4 ]
Yield Reaction Conditions Operation in experiment
85%
Example 30 Preparation of 2',7'-difluorofluorescein (29) Following general method H, Compound 29 is obtained from Compound 15 and phthalic anhydride in 85% yield. 1 H-NMR (d6 -DMSO) 7.99 (d, 1H), 7.79 (t, 1H), 7.72 (t, 1H), 7.29 (d, 1H), 6.89 (d, 2H), 6.48 (d, 2H).
Yield Reaction Conditions Operation in experiment
With oxygen UV-irradiation;
6
[ 358-23-6 ]
[ 195136-58-4 ]
2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-3',6'-diyl bis(trifluoromethanesulfonate)
[ No CAS ]
Yield Reaction Conditions Operation in experiment
82%
With pyridine In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere;
7
[ 195136-58-4 ]
3,6-diamino-9-(2-carboxyphenyl)-2,7-difluoroxanthylium trifluoroacetate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 4 h / 0 - 20 °C / Inert atmosphere
2: tris-(dibenzylideneacetone)dipalladium(0) ; caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene / 1,4-dioxane / 18 h / 100 °C / Inert atmosphere
3: dichloromethane / 2 h / 20 °C / Inert atmosphere
8
[ 195136-58-4 ]
di-tert-butyl (2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-3',6'-diyl)dicarbamate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: pyridine / dichloromethane / 4 h / 0 - 20 °C / Inert atmosphere
2: tris-(dibenzylideneacetone)dipalladium(0) ; caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene / 1,4-dioxane / 18 h / 100 °C / Inert atmosphere
9
[ 75-36-5 ]
[ 195136-58-4 ]
[ 1027558-93-5 ]
Yield Reaction Conditions Operation in experiment
With pyridine; dmap In dichloromethane for 1h;
10
[ 195136-58-4 ]
2',7'-difluoro-3'-hydroxy-6'-isopropoxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: potassium carbonate / N,N-dimethyl-formamide / 15 h / Inert atmosphere
2: sodium hydroxide / methanol; water / 15 h
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
11
[ 195136-58-4 ]
cis-2',7'-difluoro-3'-isopropoxy-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthen]-6'-yl-hexahydro-[1,2]dithiino[4,5-b]pyridine-1(2H)-carboxylate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 4 steps
1: potassium carbonate / N,N-dimethyl-formamide / 15 h / Inert atmosphere
2: sodium hydroxide / methanol; water / 15 h
3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
4: N-ethyl-N,N-diisopropylamine / dichloromethane / 2.5 h / 0 - 20 °C
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
12
[ 195136-58-4 ]
cis-2',7'-difluoro-3'-isopropoxy-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthen]-6'-yl hexahydrofuro[3,4-b]pyridine-1(2H)-carboxylate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 4 steps
1: potassium carbonate / N,N-dimethyl-formamide / 15 h / Inert atmosphere
2: sodium hydroxide / methanol; water / 15 h
3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
4: N-ethyl-N,N-diisopropylamine / dichloromethane / 2.5 h / 0 - 20 °C
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
13
[ 195136-58-4 ]
2',7'-difluoro-3'-isopropoxy-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthen]-6'-yl ((S)-1,2-dithian-4-yl)(methyl)carbamate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 4 steps
1: potassium carbonate / N,N-dimethyl-formamide / 15 h / Inert atmosphere
2: sodium hydroxide / methanol; water / 15 h
3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
4: N-ethyl-N,N-diisopropylamine / dichloromethane / 2.5 h / 0 - 20 °C
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
14
[ 195136-58-4 ]
C24 H15 ClF2 O6
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide / 15 h / Inert atmosphere
2: sodium hydroxide / methanol; water / 15 h
3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
15
[ 75-30-9 ]
[ 195136-58-4 ]
C26 H22 F2 O5
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With potassium carbonate In N,N-dimethyl-formamide for 15h; Inert atmosphere;
Reference:
[1]Becker, Katja; Busker, Sander; Felber, Jan G.; Maier, Martin S.; Poczka, Lena; Scholzen, Karoline; Theisen, Ulrike; Thorn-Seshold, Julia; Thorn-Seshold, Oliver; Zeisel, Lukas; Arnér, Elias S. J.; Brandstädter, Christina
[Journal of the American Chemical Society, 2021, vol. 143, # 23, p. 8791 - 8803]
16
chloroacetamidomethanol
[ No CAS ]
[ 195136-58-4 ]
4'-Aminomethyl Difluoro-Fluorescein
[ No CAS ]
Yield Reaction Conditions Operation in experiment
29 Synthesis of 4'-Aminomethyl Difluoro-Fluorescein (4-AMDFF)
Example 29 Synthesis of 4'-Aminomethyl Difluoro-Fluorescein (4-AMDFF) 4-AMDFF, i.e.: was prepared according to the following synthetic scheme: 0.1 g of chloroacetamidomethanol (CLAM, 0.82 mmol) in concentrated sulfuric acid (1.5 mL) was added to a solution of 2',7'-difluorofluorescein (commercially from Chemodex Ltd. of Switzerland, 0.3 g, 0.82 mmol) in sulfuric acid (4 mL). After stirring protected from light for 18 h, the reaction mixture was poured over ice (25 mL) to form an orange colored precipitate. The orange precipitate was collected from the melted ice via filtration, washed with water, and dried to yield 0.3 g of product.
17
C29 H27 N5 O5
[ No CAS ]
[ 195136-58-4 ]
C49 H35 F2 N5 O9
[ No CAS ]
Yield Reaction Conditions Operation in experiment
2 mg
Stage #1: C29 H27 N5 O5 With 4-dimethylaminopyridine In N,N-dimethyl-formamide at 20℃; for 0.5h;
Stage #2: 2',7'-difluorofluorescein In N,N-dimethyl-formamide at 20℃;
11 Production Example 11 Synthesis of Compound 6
12 mg of the compound 1-2, 16 mg of water-soluble carbodiimide, and 3 mg of dimethylaminopyridine were added to 1 mL of N,N-dimethylformamide, and the mixture was stirred at room temperature for 30 minutes. 10 mg of 2′,7′-difluorofluorescein (manufactured by Thermo Fisher Scientific Inc.) was added to the mixture, and the mixture was stirred overnight at room temperature. After the solvent was distilled off, the mixture was purified by silica gel column chromatography. Thus, 2 mg of a red solid was prepared.

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping