Alternatived Products of [ 199125-21-8 ]
Product Details of [ 199125-21-8 ]
| CAS No. : | 199125-21-8 |
MDL No. : | MFCD21667125 |
| Formula : |
C7H10N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
138.17
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 199125-21-8 ]
Application In Synthesis of [ 199125-21-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 199125-21-8 ]
- 1
-
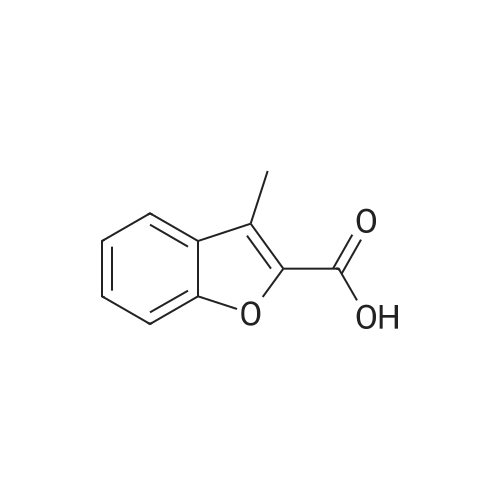 [ 24673-56-1 ]
[ 24673-56-1 ]

-
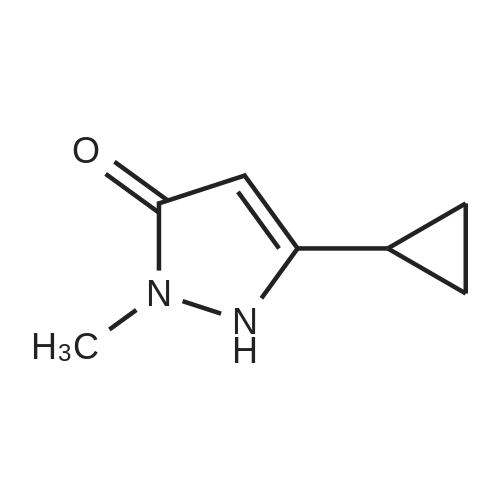 [ 199125-21-8 ]
[ 199125-21-8 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 3-methylbenzofuran-2-formate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 0.55 g |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 10 - 30℃;Inert atmosphere; |
3-methylbenzofuran-2-carboxylic acid (0.50 g), 3-cyclopropyl-1-methylpyrazolol (0.39 g), and 4-dimethylaminopyridine (DMAP 0.05 g) obtained in Example 3 ),1-Ethyl- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI 0.59 g) and 50 mL of anhydrous dichloromethane were added to a 100 mL single-necked flask,Turn on the nitrogen protection system, react at room temperature, and control the TLC until the reaction is complete.Product dissolution,The compound (0.55 g) was separated and purified by silica gel column chromatography. |
- 2
-
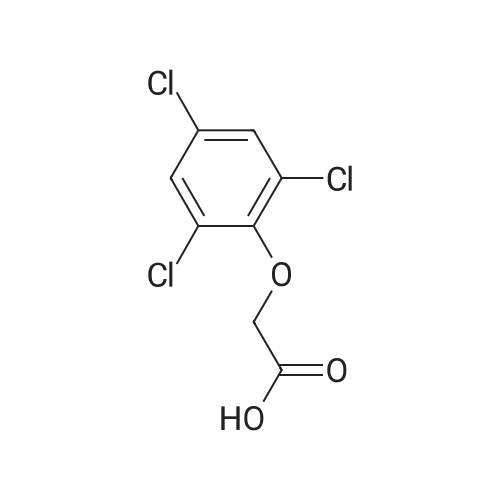 [ 575-89-3 ]
[ 575-89-3 ]

-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-(2,4,6-trichlorophenoxy)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃;Inert atmosphere; |
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
|
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃; for 12h; |
In a 50ml round bottom flask,Dissolve 1.5 g of I-3 in 30 ml of dry DCM,0.73g of 3-cyclopropyl-1-methyl-1H-pyrazole-5-ol,1.3g EDCI and 0.02g DMAP,Stir at room temperature for 12h, stop the reaction, separate and purify by column chromatography,Compound A-24 was obtained. |
- 3
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
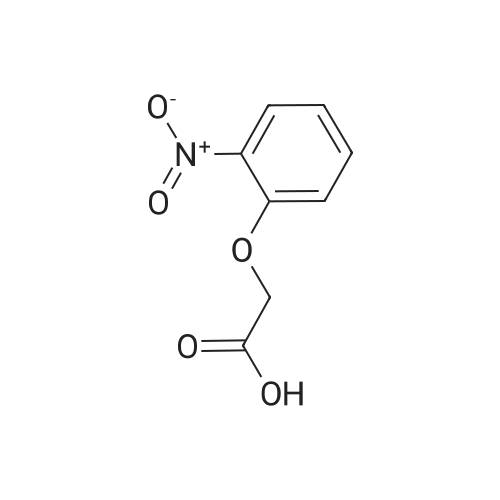 [ 1878-87-1 ]
[ 1878-87-1 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-(2-nitrophenoxy)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 60% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; |
General procedure for intermediate E1 - E40
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Reference:
[1]Huang, Hao; Liu, Jian-Min; Shu, Lei; Wang, Man-Man; Yan, Yi-Le; Zhang, Da-Yong; Zhang, Jian-Qiu
[Beilstein Journal of Organic Chemistry, 2020, vol. 16, p. 233 - 247]
- 4
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
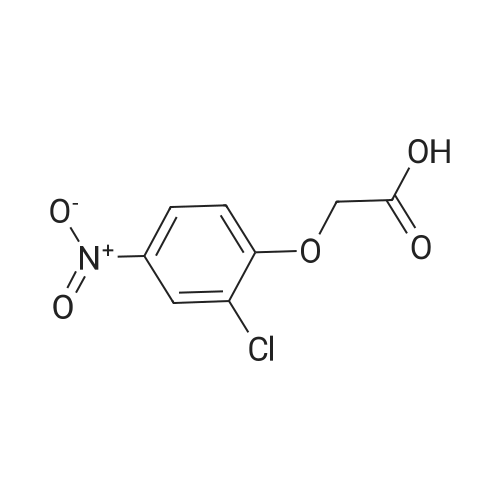 [ 5037-04-7 ]
[ 5037-04-7 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-(2-chloro-4-nitrophenoxy)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 64% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; |
General procedure for intermediate E1 - E40
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Reference:
[1]Huang, Hao; Liu, Jian-Min; Shu, Lei; Wang, Man-Man; Yan, Yi-Le; Zhang, Da-Yong; Zhang, Jian-Qiu
[Beilstein Journal of Organic Chemistry, 2020, vol. 16, p. 233 - 247]
- 5
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
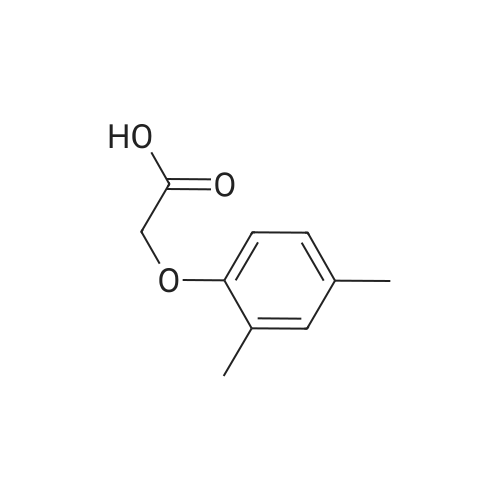 [ 13334-49-1 ]
[ 13334-49-1 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-(2,4-dimethylphenoxy)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 54% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; |
General procedure for intermediate E1 - E40
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Reference:
[1]Huang, Hao; Liu, Jian-Min; Shu, Lei; Wang, Man-Man; Yan, Yi-Le; Zhang, Da-Yong; Zhang, Jian-Qiu
[Beilstein Journal of Organic Chemistry, 2020, vol. 16, p. 233 - 247]
- 6
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
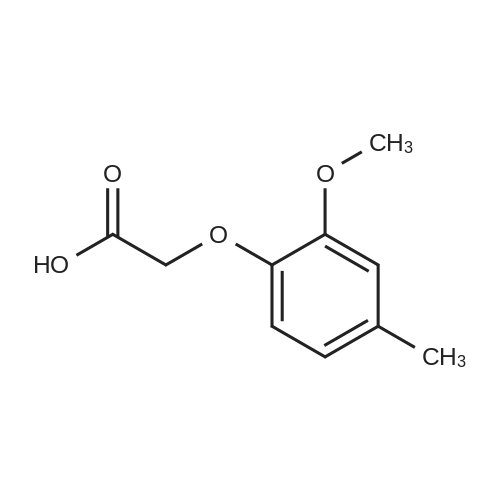 [ 6270-23-1 ]
[ 6270-23-1 ]

-
C17H20N2O4
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; |
General procedure for intermediate E1 - E40
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Reference:
[1]Huang, Hao; Liu, Jian-Min; Shu, Lei; Wang, Man-Man; Yan, Yi-Le; Zhang, Da-Yong; Zhang, Jian-Qiu
[Beilstein Journal of Organic Chemistry, 2020, vol. 16, p. 233 - 247]
- 7
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
 [ 55335-06-3 ]
[ 55335-06-3 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-((3,5,6-trichloropyridin-2-yl)oxy)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 64% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃;Inert atmosphere; |
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
- 8
-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
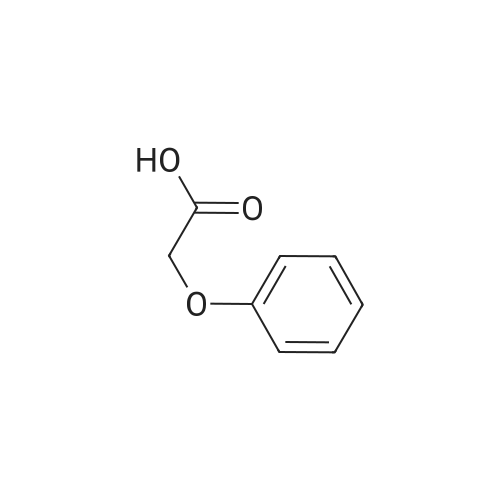 [ 122-59-8 ]
[ 122-59-8 ]

-
3-cyclopropyl-1-methyl-1H-pyrazol-5-yl 2-phenoxyacetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; |
General procedure for intermediate E1 - E40
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Reference:
[1]Huang, Hao; Liu, Jian-Min; Shu, Lei; Wang, Man-Man; Yan, Yi-Le; Zhang, Da-Yong; Zhang, Jian-Qiu
[Beilstein Journal of Organic Chemistry, 2020, vol. 16, p. 233 - 247]
- 9
-
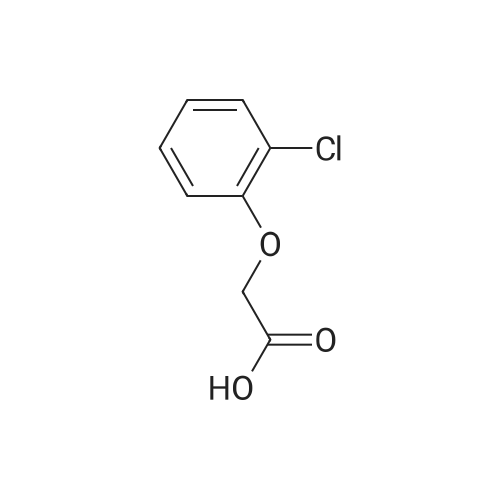 [ 614-61-9 ]
[ 614-61-9 ]

-
 [ 199125-21-8 ]
[ 199125-21-8 ]

-
C15H15ClN2O3
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃;Inert atmosphere; |
General procedure: In the presence of DMAP as the catalyst to speed up the reaction, substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazol-5-ol (0.1 mol), EDCI (0.1mol), anhydrous dichloromethane (DCM) (30 mL) and compound D (0.1 mol) wereadded to a 50 mL eggplant-shaped. The solution was stirred for 5-8 h. The progress ofthe reaction was monitored by TLC. After completion of the reaction, DCM was removed from the system under reduced pressure. Residues were purified via flashchromatography (Vethylacetate: Vpetroleumether =1:3) to afford the enol ester. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping













