Alternatived Products of [ 203790-61-8 ]
Product Details of [ 203790-61-8 ]
| CAS No. : | 203790-61-8 |
MDL No. : | MFCD22124353 |
| Formula : |
C15H19NO5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
293.32
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 203790-61-8 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 203790-61-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 203790-61-8 ]
- 1
-
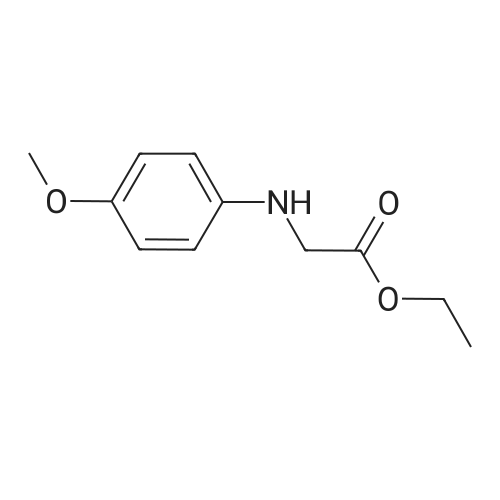 [ 50845-77-7 ]
[ 50845-77-7 ]

-
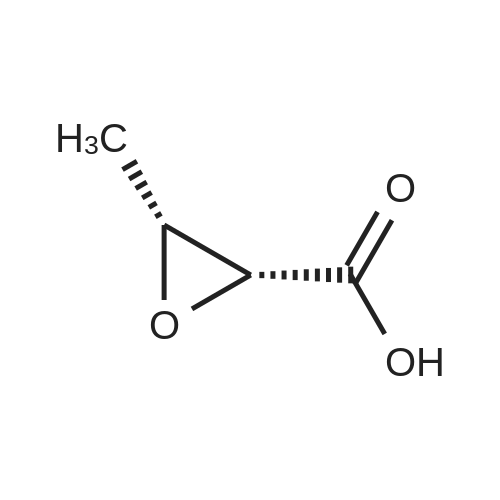 [ 86561-72-0 ]
[ 86561-72-0 ]

-
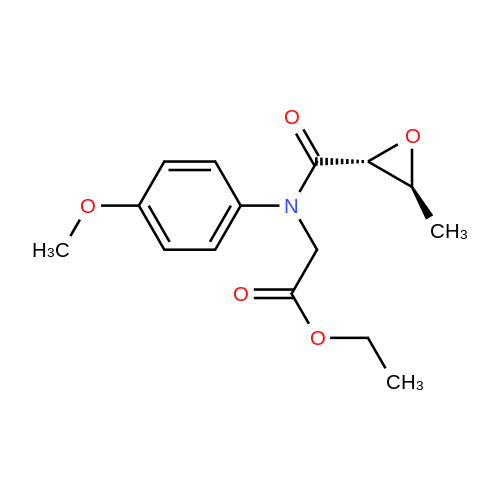 [ 203790-61-8 ]
[ 203790-61-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With dicyclohexyl-carbodiimide In dichloromethane at 0 - 25℃; for 2.5h; |
3
Example 3: Preparation of f2RJR)-N-fefcoxycarbonyl)methyl-N-p-methoxyphenyl-2,3- epoxybutyric amido; To a stirred solution of (2R,3R)-epoxybutyric acid (50 g) in dichloromethane, was added ethyl N-p-methoxyphenyl glycinate (92.2 g) at 0-50C. A solution of dicyclohexylcarbodiimide (101 g) in dichloromethans (75 ml) was added to the reaction mass in 1.5 hours at 0-50C. To this reaction mixture, waier (75 ml) was added at 0-50C followed by stirring at 20-250C for 1 hour. The reaction mass was filtered to remove solids. The solids were washed with dichloromethane (2 x 75 ml) and the washings added to the filtrate. The filtrate was washed with 2 x 150 ml 3N HCl and 1 x 50 ml saturated sodium bicarbonate solution at 15°C. Dichloromethane was recovered under vacuum to obtain the title compound as a thick oil which can be used as such in the next step. EPO Yield =145 g |
|
Stage #1: (2R,3R)-2,3-epoxybutanoic acid With trichlorophosphate In dichloromethane at -25℃; for 1h;
Stage #2: N-(4-methoxy)phenylglycine ethyl ester With triethylamine In dichloromethane at 20℃; |
2.3 Step three:N- (4-methoxyphenyl) -2-[(2'R, 3'R) -epoxybutyramido] -2-acetic acidEthyl esterPreparation
Add 500g of dichloromethane and 88g of epoxybutyric acid (1.3eq) to a 1000ml reaction bottle.Reduce the temperature to -25 ± 2 , add 183g (1.8eq) of phosphorus oxychloride, and heat it at -25 ± 2 for 1 hour.After incubation, 138.0 g (1.0 eq) of ethyl N- (4-methoxyphenyl) -2-aminoacetate and 132 g (2.0 eq) of triethylamine were added.The temperature was raised to 20 ° C, and the reaction was held for 5 to 8.0 hours, and then poured into a 1200 ml flask containing 2N hydrochloric acid.Stir to separate layers, wash the organic layer again with 250ml of 6% NaHCO3 solution,After washing with 10% NaCl solution, the organic layer was dried by adding 50 g of anhydrous magnesium sulfate, filtered, and concentrated.145 g of N- (4-methoxyphenyl) -2-[(2'R, 3'R) -epoxybutyramido] -2-ethyl acetate was obtained,Yield: 74.2%, HPLC = 94.5%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping





