Alternatived Products of [ 20624-92-4 ]
Product Details of [ 20624-92-4 ]
| CAS No. : | 20624-92-4 |
MDL No. : | MFCD00016870 |
| Formula : |
C8H9ClO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
188.61
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 20624-92-4 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 20624-92-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 20624-92-4 ]
- 1
-
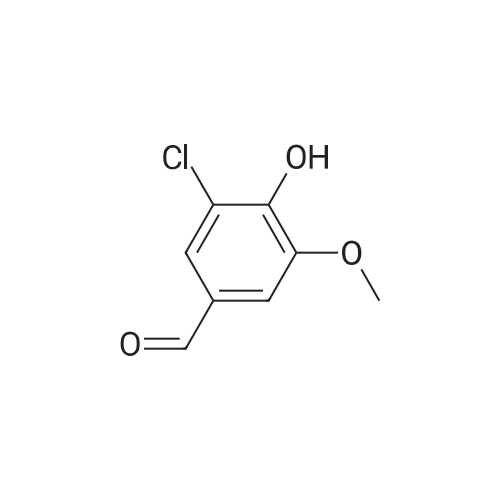 [ 19463-48-0 ]
[ 19463-48-0 ]

-
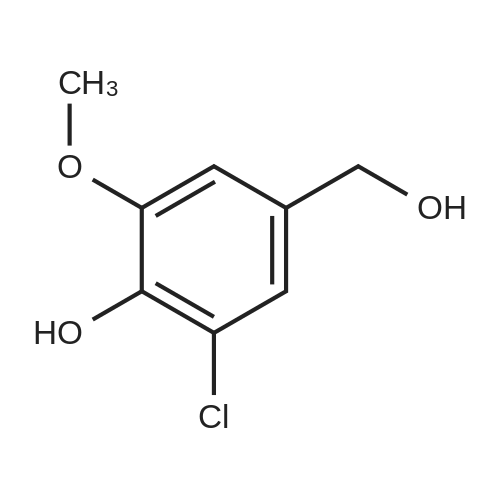 [ 20624-92-4 ]
[ 20624-92-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydroxide; sodium tetrahydroborate |
|
|
With sodium hydroxide; sodium tetrahydroborate |
|
|
With sodium tetrahydroborate; water In acetonitrile |
|
Reference:
[1]Smith
[Analytical Chemistry, 1958, vol. 30, p. 150]
[2]Claus,P. et al.
[Monatshefte fur Chemie, 1972, vol. 103, p. 1178 - 1193]
[3]Pratihar, Sanjay
[Organic and Biomolecular Chemistry, 2014, vol. 12, # 30, p. 5781 - 5788]
- 2
-
 [ 20624-92-4 ]
[ 20624-92-4 ]

-
 [ 19463-48-0 ]
[ 19463-48-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
With potassium carbonate In n-heptane at 80℃; for 24h; |
S4. Procedure for the synthesis of aldehydes and ketones
General procedure: A magnetic stir bar, 0.5 mmol alcohol and 3 mL n-heptane solvent were added to 20 mL glass tube. Then, 35mg catalyst and 10 mol% of K2CO3 were added. The glass tube containing reaction mixture was f itted withseptum and connected to a balloon containing one bar of air. Then the glass tube was placed into a preheatedaluminum block at 85°C. Temperature inside the reaction tube was measured to be 80 oC and this temperaturehas been taken as the reaction temperature. The reaction was allowed to progress under continuous stirringfor the required time at 80 °C. Af ter completion of the reaction, the glass tube was cooled down to roomtemperature. Afterwards, the catalyst was f iltered-off and washed with ethyl acetate. The solvent f rom thef iltrate containing the reaction products was removed in vacuum and the corresponding aldehyde/ketone waspurif ied by column chromatography. All products were analyzed by GC-MS and NMR spectroscopy analysis.In the case of yields determined the by GC, 100 μL n-hexadecane was added to the reaction vial containingthe products and diluted with ethyl acetate. Then, the reaction mixture containing catalyst and products wasf iltered through a plug of silica and the filtrate containing product was analyzed by GC. |
|
With oxygen; copper(l) chloride In dimethyl sulfoxide |
|
Reference:
[1]Bartling, Stephan; Beller, Matthias; Chandrashekhar, Vishwas G.; Jagadeesh, Rajenahally V.; Rabeah, Jabor; Rockstroh, Nils; Senthamarai, Thirusangumurugan
[Chem, 2022, vol. 8, # 2, p. 508 - 531]
[2]Claus,P. et al.
[Monatshefte fur Chemie, 1972, vol. 103, p. 1178 - 1193]
- 3
-
 [ 19463-48-0 ]
[ 19463-48-0 ]

-
 [ 20624-92-4 ]
[ 20624-92-4 ]

-
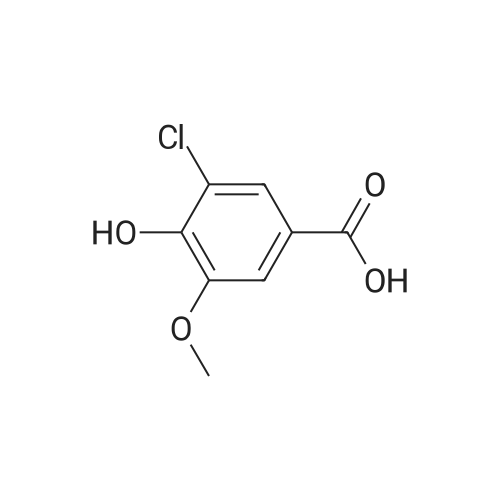 [ 62936-23-6 ]
[ 62936-23-6 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping





