Alternatived Products of [ 22966-22-9 ]
Product Details of [ 22966-22-9 ]
| CAS No. : | 22966-22-9 |
MDL No. : | |
| Formula : |
C15H11ClO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
242.70
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 22966-22-9 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 22966-22-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 22966-22-9 ]
- 1
-
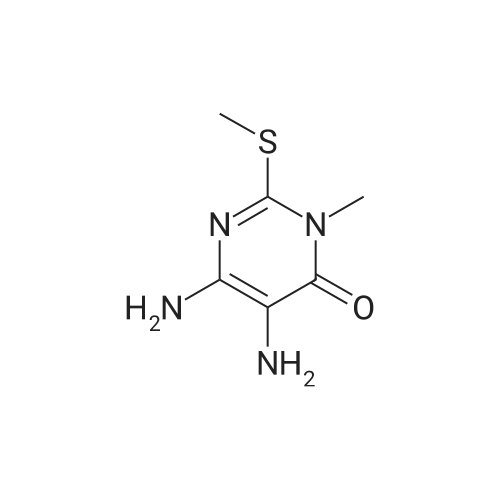 [ 39008-28-1 ]
[ 39008-28-1 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
4-(4-chlorophenyl)-2,3,6,7-tetrahydro-7-methyl-8-methylthio-2-phenyl-1H-pyrimido<4,5-b><1,4>diazepin-6-one
[ No CAS ]
- 2
-
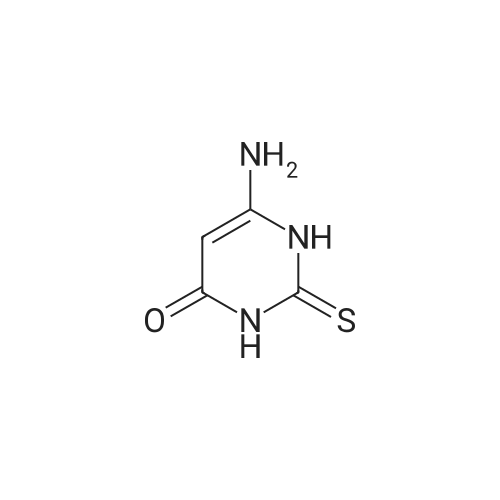 [ 1004-40-6 ]
[ 1004-40-6 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
 [ 150356-87-9 ]
[ 150356-87-9 ]
- 3
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
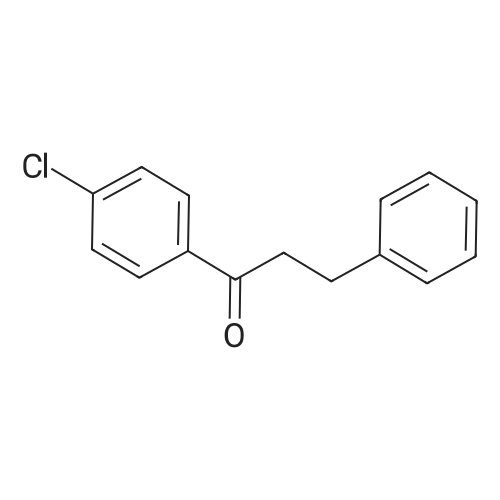 [ 5739-37-7 ]
[ 5739-37-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98% |
With hydrogen In methanol for 20h; |
|
| 98% |
With hydrogen In methanol at 20℃; for 20h; |
|
| 98% |
With hydrogen In methanol at 20℃; for 24h; |
|
| 97% |
With diphenyl sulfide; hydrogen In methanol at 20℃; for 24h; |
|
| 97% |
With diphenyl sulfide; hydrogen In methanol at 20℃; for 24h; |
|
| 96% |
With ammonium acetate; zinc In ethanol at 20℃; for 1.25h; |
|
| 92% |
With 9-borabicyclo[3.3.1]nonane dimer; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In ethyl acetate at 40℃; for 16h; |
|
| 90% |
With sodium tetrahydroborate; nickel dichloride In methanol; water at 20℃; for 0.5h; |
|
| 90% |
With ammonium chloride; zinc In ethanol; water at 20℃; for 0.75h; |
|
| 86% |
With magnesium; zinc(II) chloride for 0.416667h; |
|
|
Multi-step reaction with 2 steps
1: sodium tetrahydroborate; cerium(III) chloride monohydrate / methanol / 0.17 h
2: sodium t-butanolate; 1,10-Phenanthroline / toluene / 2 h / 80 °C / Inert atmosphere |
|

Reference:
[1]Sajiki, Hironao; Ikawa, Takashi; Yamada, Hiromi; Tsubouchi, Kozo; Hirota, Kosaku
[Tetrahedron Letters, 2003, vol. 44, # 1, p. 171 - 174]
[2]Ikawa, Takashi; Sajiki, Hironao; Hirota, Kosaku
[Tetrahedron, 2005, vol. 61, # 8, p. 2217 - 2231]
[3]Kitamura, Yoshiaki; Tanaka, Asami; Sato, Mutsumi; Oono, Keiji; Ikawa, Takashi; Maegawa, Tomohiro; Monguchi, Yasunari; Sajiki, Hironao
[Synthetic Communications, 2007, vol. 37, # 24, p. 4381 - 4388]
[4]Mori, Akinori; Miyakawa, Yumi; Ohashi, Eri; Haga, Tomoko; Maegawa, Tomohiro; Sajiki, Hironao
[Organic Letters, 2006, vol. 8, # 15, p. 3279 - 3281]
[5]Mori, Akinori; Mizusaki, Tomoteru; Miyakawa, Yumi; Ohashi, Eri; Haga, Tomoko; Maegawa, Tomohiro; Monguchi, Yasunari; Sajiki, Hironao
[Tetrahedron, 2006, vol. 62, # 51, p. 11925 - 11932]
[6]Zhou, Yong-Bo; Wang, Yu-Iu; Wang, Jin-Ye
[Journal of Chemical Research, 2004, # 2, p. 118 - 119]
[7]Nicholson, Kieran; Langer, Thomas; Thomas, Stephen P.
[Organic Letters, 2021, vol. 23, # 7, p. 2498 - 2504]
[8]Khurana, Jitender Mohan; Sharma, Purnima
[Bulletin of the Chemical Society of Japan, 2004, vol. 77, # 3, p. 549 - 552]
[9]Li, Jian-Ping; Zhang, Yong-Xia; Ji, Yan
[Journal of the Chinese Chemical Society, 2008, vol. 55, # 2, p. 390 - 393]
[10]Saikia, Anil; Barthakur, Madan Gopal; Boruah, Romesh Chandra
[Synlett, 2005, # 3, p. 523 - 525]
[11]Zheng, Hong-Xing; Xiao, Zu-Feng; Yao, Chuan-Zhi; Li, Qiang-Qiang; Ning, Xiao-Shan; Kang, Yan-Biao; Tang, Yong
[Organic Letters, 2015, vol. 17, # 24, p. 6102 - 6105]
- 4
-
 [ 1004-40-6 ]
[ 1004-40-6 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
 [ 150356-90-4 ]
[ 150356-90-4 ]
- 5
-
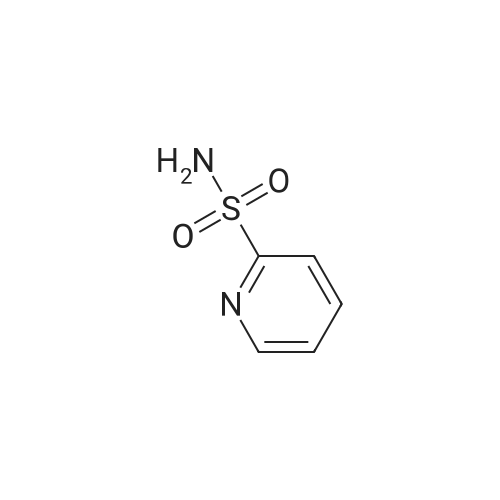 [ 63636-89-5 ]
[ 63636-89-5 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
(E)-1-(4-chlorophenyl)-3-phenyl-N-[(2-pyridyl)sulfonyl]prop-2-en-1-imine
[ No CAS ]
- 6
-
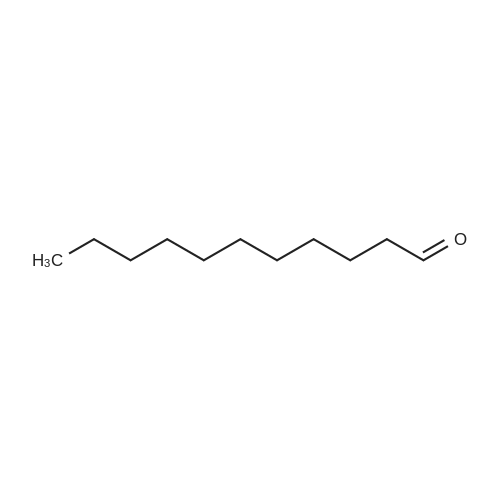 [ 112-44-7 ]
[ 112-44-7 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
1-(4-chlorophenyl)-3-phenyltetradecane-1,4-dione
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 97% |
With triethylamine In N,N-dimethyl-formamide at 80℃; for 22h; |
|
- 7
-
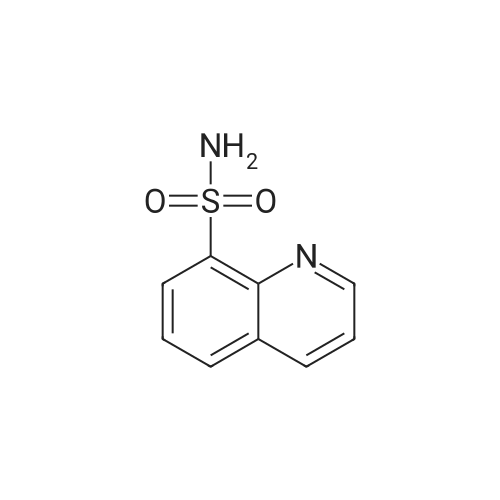 [ 35203-91-9 ]
[ 35203-91-9 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
(E)-1-(4-chlorophenyl)-3-phenyl-N-[(8-quinoyl)sulfonyl]prop-2-en-1-imine
[ No CAS ]
- 8
-
 [ 7677-24-9 ]
[ 7677-24-9 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
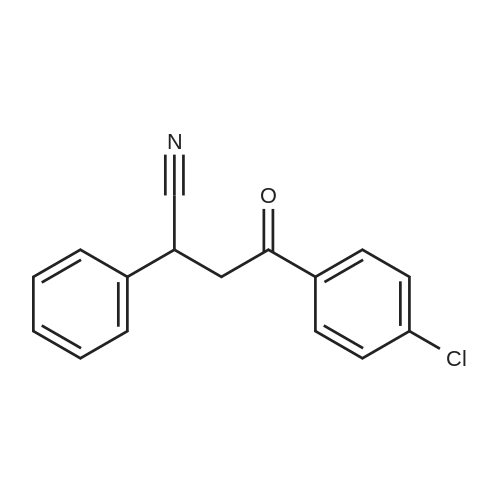 [ 6273-45-6 ]
[ 6273-45-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 72% |
Stage #1: trimethylsilyl cyanide; (E)-1-(4-chlorophenyl)-3-phenyl-2-propen-1-one for 0.0833333h; microwave irradiation;
Stage #2: With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 0.0833333h; |
|
- 9
-
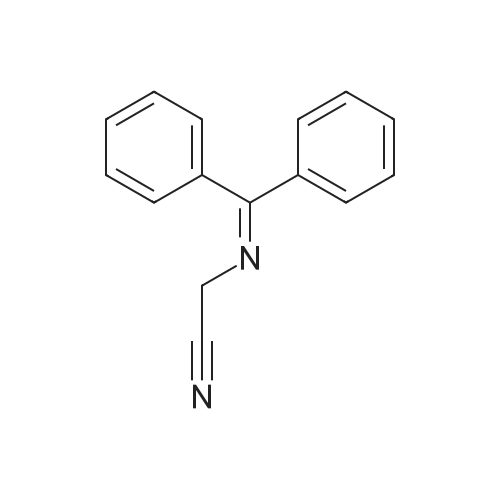 [ 70591-20-7 ]
[ 70591-20-7 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
C30H23ClN2O
[ No CAS ]
- 10
-
 [ 98-54-4 ]
[ 98-54-4 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
6-tert-butyl-2-(4-chlorophenyl)-4-phenyl-2H-chromen-2-ol
[ No CAS ]

-
 [ 5739-37-7 ]
[ 5739-37-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 69%
2: 68% |
With trifluoroacetic acid for 72h; Heating; |
|
- 11
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
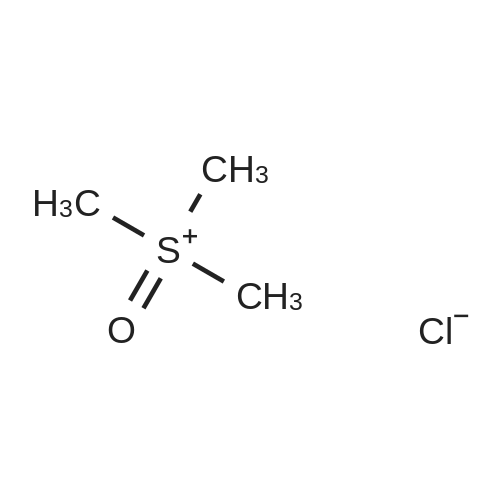 [ 5034-06-0 ]
[ 5034-06-0 ]

-
C16H13ClO
[ No CAS ]

-
 [ 959936-89-1 ]
[ 959936-89-1 ]
- 12
-
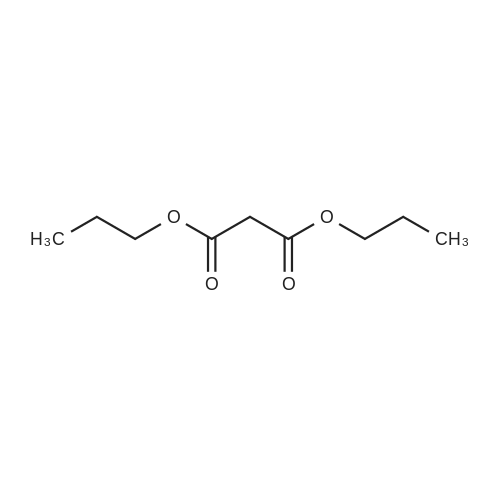 [ 1117-19-7 ]
[ 1117-19-7 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
 [ 1012919-08-2 ]
[ 1012919-08-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98% |
With 4 A molecular sieve In toluene at 25℃; for 7h; |
|
- 13
-
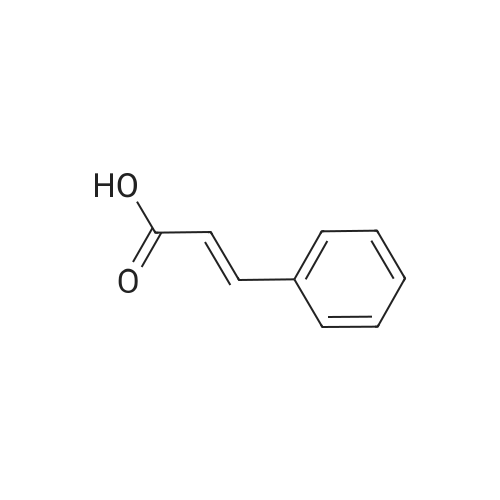 [ 140-10-3 ]
[ 140-10-3 ]

-
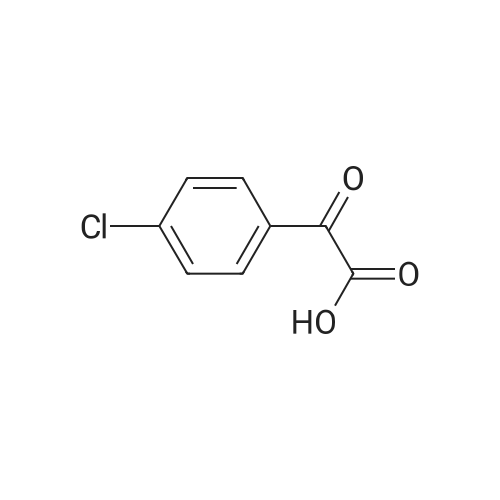 [ 7099-88-9 ]
[ 7099-88-9 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]
- 14
-
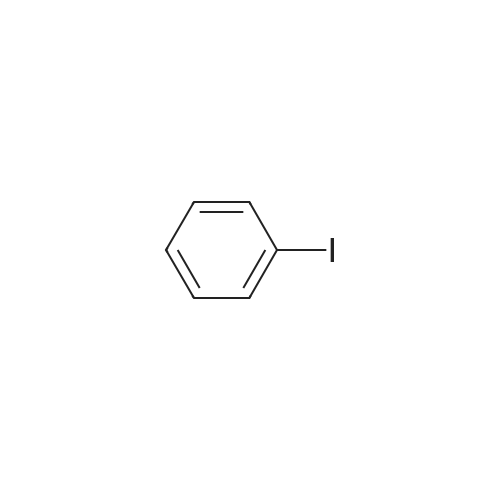 [ 591-50-4 ]
[ 591-50-4 ]

-
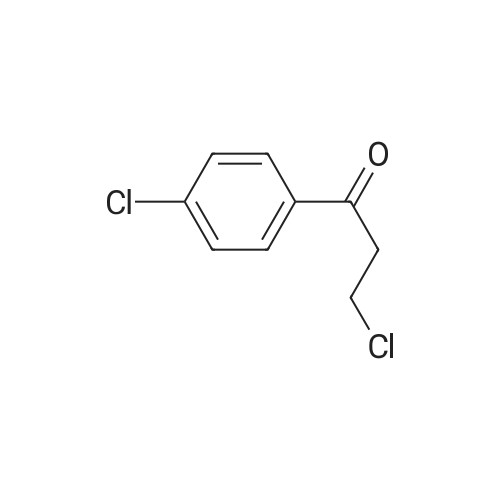 [ 3946-29-0 ]
[ 3946-29-0 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With palladium diacetate; potassium carbonate; triphenylphosphine In N,N-dimethyl-formamide at 20 - 90℃; for 16h; Inert atmosphere; |
General procedure for the synthesis of chalcones - synthesis of chalcone 3a
General procedure: A mixture of Pd(OAc)2 (4.5 mg, 0.02 mmol), PPh3 (11.2 mg, 0.04 mmol), iodobenzene (1a) (82 mg, 0.4mmol), 3-chloropropiophenone (2a) (87 mg, 0.5 mmol), and K2CO3 (166 mg, 1.2 mmol) in DMF (2.5 mL) was stirred under a N2 atmosphere at room temperature for 10 min, and then heated at 90 °C for 16 h. The reaction was then cooled to ambient temperature and diluted with CH2Cl2 (10 mL) before being filtered through a short pad of silica gel. The silica pad was rinsed with DCM (5 mL), and the combined filtrates were washed with brine (15 mL), dried over anhydrous Na2SO4. The solvent was then removed under reduced pressure to give the crude product as a residue, which was purified by silica gel column chromatography eluting with a mixture of petroleum ether (60-90 °C)/EtOAc (v/v = 30:1). |
- 15
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
 [ 67-68-5 ]
[ 67-68-5 ]

-
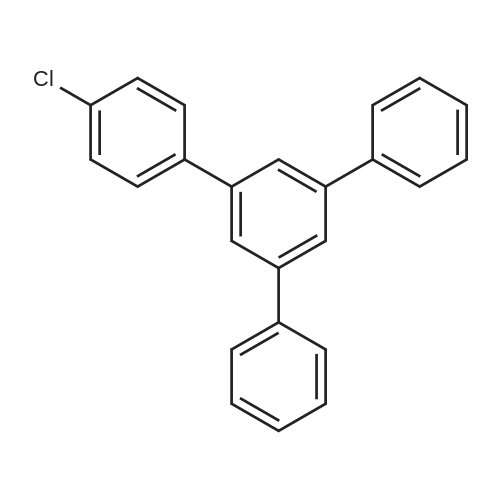 [ 116941-51-6 ]
[ 116941-51-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 51% |
With sodium t-butanolate at 60℃; for 5h; |
5 Preparation of 1- (4-chlorophenyl) -3,5-diphenylbenzene
mmol of 4'-chlorchalcone, 1.5 mmol of t-butoxide and 2.0 mL of dimethylsulfoxide were added to 10 mL ofShould be placed in the oil bath at 60 , the exposure in the air reaction 5h. The reaction was stopped and allowed to cool to room temperature. The reaction solution is usedDiluted with ethyl acetate and extracted three times with water. The organic phase was dried over anhydrous Na2SO4, filtered and chromatographed to give 20.6Mg of the target product in a yield of 51%. |
- 16
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
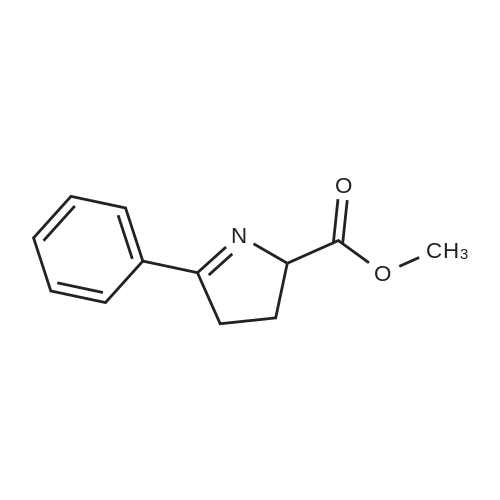 [ 111836-35-2 ]
[ 111836-35-2 ]

-
(S)-methyl 2-((R)-3-(4-chlorophenyl)-3-oxo-1-phenylpropyl)-5-phenyl-3,4-dihydro-2H-pyrrole-2-carboxylate
[ No CAS ]

-
C27H24ClNO3
[ No CAS ]
- 17
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
 [ 111836-35-2 ]
[ 111836-35-2 ]

-
C27H24ClNO3
[ No CAS ]
- 18
-
 [ 22966-22-9 ]
[ 22966-22-9 ]

-
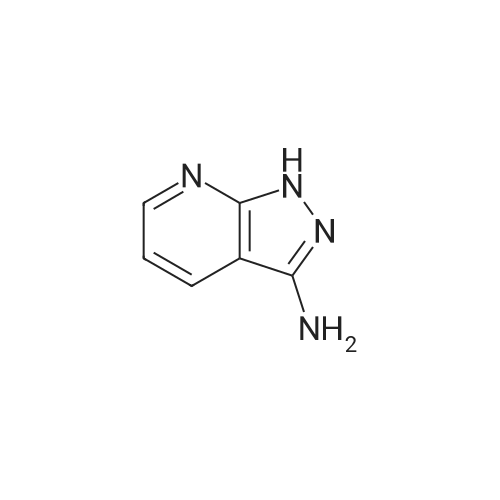 [ 6752-16-5 ]
[ 6752-16-5 ]

-
2-(4-chlorophenyl)-4-phenylpyrido[2’,3’:3,4]pyrazolo[1,5-a]pyrimidine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 90% |
With cholin hydroxide; In neat (no solvent); at 80℃; for 0.833333h;Green chemistry; |
General procedure: ChOH (1 mmol) was added to a mixture of <strong>[6752-16-5]1H-pyrazolo[3,4-b]pyridin-3-amine</strong> (1,1.0 mmol) and (2E)-1,3-diphenylprop-2-en-1-one (2a, 1.0 mmol) in a 10-mL reaction flask equipped with a magnetic stirrer. The resulting mixture was stirred for the appropriate time at 80 C. After completion of the reaction (confirmed by TLC, hexane:EtOAc 1:1), 2 mL of water was added and stirred 60 for minutes at room temperature. The solid product was filtered and washed with water. The obtained crude product was recrystallized from ethanol to yield the pure product 3a. The same method was adopted for the synthesis of all the targeted products 3a-3aj. |
- 19
-
 [ 5739-37-7 ]
[ 5739-37-7 ]

-
 [ 22966-22-9 ]
[ 22966-22-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98% |
With iron(III) chloride; 1,10-Phenanthroline; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In dimethyl sulfoxide at 120℃; for 24h; Inert atmosphere; Schlenk technique; |
|
Reference:
[1]Zhang, Xiao-Wei; Jiang, Guo-Qing; Lei, Shu-Hui; Shan, Xiang-Huan; Qu, Jian-Ping; Kang, Yan-Biao
[Organic Letters, 2021, vol. 23, # 5, p. 1611 - 1615]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping























