|
In toluene;Reflux; Dean-Stark; |
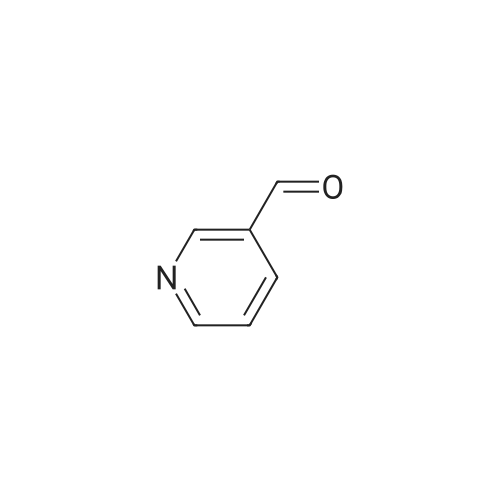
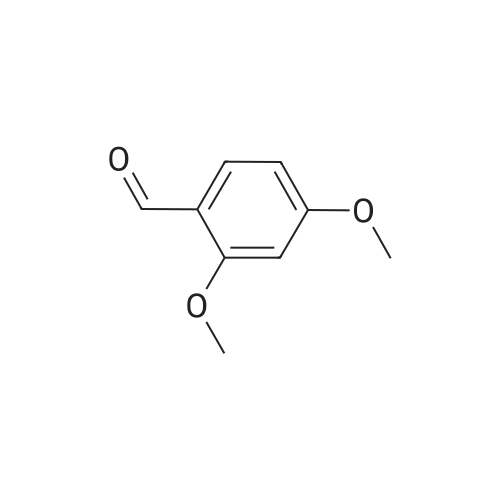
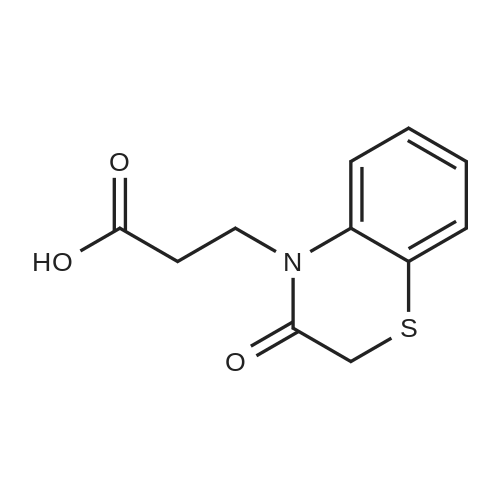
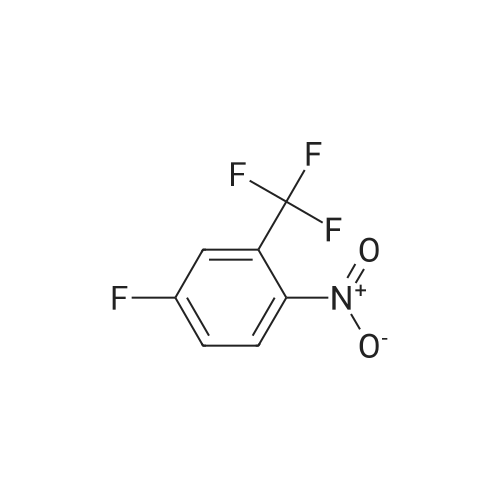
2,4-Dimethoxybenzaldehyde (3.8 g) and compound 26 (5.3 g) in toluene (100 ml) were refluxed with Dean-Stark trap until no water separated. After cooling, toluene was evaporated on rotary evaporator. The obtained compound 27 was used further without purification. To a solution of compound 27 in methanol (50 ml) at stirring NaBH4 (1.3 g) was added, the mixture was stirred at room temperature for 1 h, methanol was evaporated on rotary evaporator, water (50 ml) was added, rendered alkaline with 10% solution of KOH to pH=9, extracted with methylene chloride, dried, evaporated. It gave compound 28, yield 80%. To a solution of compound 9 (2.4 g) in THF (100 ml) in argon atmosphere at stirring N-hydroxysuccinimide (1.0 g) and dicyclohexylcarbodiimide (1.1 g) were added. The mixture was stirred for night, after that compound 28 (3 g) was added and mixture was stirred for 6 h at 60C. Precipitated solid was filtered off, mother solution was evaporated on rotary evaporator. The residue was purified by column chromatography, eluent - chloroform : methanol 19:1. It gave compound 29, yield 70%. A mixture of p-tolylacetic acid (10.0 g), N-bromosuccinimide (13.0 g) and 2,2'-azabisisobutyronitrile (0.1 g) in carbon tetrachloride (60 ml) was refluxed for 4 h. The mixture was cooled to room temperature, poured out on water (100 ml), precipitated solid was filtered off, and dried. To a solution of the above solid in ethanol (50 ml) at 0C SOCl2 (3.7 ml) was added, the mixture was stirred at night at room temperature, solvent was evaporated. It gave compound 30, yield 50%. The obtained product was used further without purification. Potassium carbonate (2.2 g) and ethyl ether of p-bromomethylphenylacetic acid 30 (2.0 g) were added one after another to a solution of compound 29 (4.5 g) in DMF (50 ml).The mixture was stirred at 50C for night, DMF was evaporated on rotary evaporator, water (100 ml) was added to the residue. Precipitated solid was filtered off, washed with water and dried. It gave compound 31, yield 80%. Trifluoroacetic acid (2.0 g) was added to a solution of compound 31 (3.5 g) in methylene chloride (40 ml), the resultant mixture was refluxed for night. After that the mixture was shaken up several times with conc. solution of NaHCO3, dried, methylene chloride was evaporated on rotary evaporator. The product was purified by column chromatography, eluent - chloroform : methanol 40:1. It gave compound 32, yield 55%. LiOH (0.29 g) was added to a solution of compound 32 (1.6 g) in 50% aqueous ethanol (20 ml) and stirred at room temperature for night. Then ethanol was evaporated on rotary evaporator, water solution was acidified with 10% HCl to pH=3. Precipitated solid was filtered off, washed with water and dried. The product was purified by column chromatography, eluent - chloroform : methanol : triethylamin 10:1:1. It gave compound 33, yield 15%. N-hydroxysuccinimide (47 mg) and dicyclohexylcarbodiimide (84 mg) were added to a solution of compound 33 (180 mg) in THF (20 ml), in argon atmosphere and at stirring. Mixture was stirred at room temperature for night, precipitated solid was filtered off, mother solution was evaporated, product was purified by flash chromatography on silica (eluent - ethyl acetate). It gave compound 1(5), yield 50%. NMR Spectrum of compound 1(5) is given in Fig. 5. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping